ISIS mini-series New Age of Water
Water associated with collagen shows a surprising degree of order. Could they be the super-conducting channels that enable every single cell within the body to intercommunicate for perfect coordination? Dr. Mae-Wan Ho
Collagen is the main protein in connective tissues of animals and the most abundant protein in mammals. Connective tissues contain a lot of water; soft connective tissues (all apart from bones and cartilage) are typically 60 to 70 percent of water by weight. The proteins together with the water form a liquid crystalline matrix in which every single cell in the body is embedded, which makes connective tissues the ideal medium for intercommunication, as I have suggested in my book The Rainbow And The Worm [1] .
In traditional Chinese medicine, a system of acupuncture meridians are supposed to transport qi or living energy to every part of the body, but all attempts to locate the meridians to anatomical structures have failed. A colleague and I proposed in 1998 that the acupuncture meridians may be the structured water aligned with collagen in the connective tissues [2] (The Acupuncture System and The Liquid Crystalline Collagen Fibres ...), and that the qi may be positive electric currents carried by the jump-conduction of protons through the hydrogen bonds of the water molecules aligned along the collagen fibres. Evidence for the idea has been accumulating since [3] (Acupuncture, Coherent Energy and Liquid Crystalline Meridians).
A number of observations and molecular dynamic simulations confirm that water molecules organised in hydrogen-bonded chains can act as ‘proton wires’ to support jump conduction of protons much faster than the ordinary flow of electrons through wires [4] (Non-Thermal Electromagnetic Field Effects). Meanwhile, the structure of water associated with collagen is yielding to X-ray diffraction studies on both native and synthetic collagens. But the precise organisation of the water molecules around the protein molecules remains unknown.
Gary Fullerton and colleagues at Texas University San Antonio in the United States have now produced a model based on results suggesting that the water associated with collagen is ordered to a high degree [5, 6]. The water appears to be structured in regular chains along the collagen fibrils, which would indeed facilitate the proton jump-conduction that enables every part of the body - from molecules to cells and tissues - to intercommunicate for perfect coordination. So perfect that I have describe the coordinated activities over many orders of magnitude of space and time as Quantum Jazz [7] (this issue).
Tendons have high concentrations of type I collagen, in some instances approaching 100 percent of the dry weight, which makes it easy to purify and crystallise for structural studies, and a great deal is already known.
The collagen molecule is a rod about 300 nm long and 1.5 nm in diameter, made up of three polypeptide subunits wrapped around one another in a triple helix. Collagen molecules spontaneously self-assemble end to end into long fibrils, fibrils aggregate into fibres, fibres into larger bundles, or sheets. A distinctive feature of collagen is the repeat of three amino acid subunits along the polypeptide chain Gly-X-Y, where X is frequently Proline and Y, hydroxyproline, which accounts for the tendency of three polypeptide strands to form a triple helix, with glycine in the interior of the helix, and the rings of proline and hydroxyproline stacked and pointing outward.
Type I collagen has a mean molecular weight per amino acid residue of 91.2 Daltons (a Dalton is a unit of mass equivalent to 1 g), which is calculated from the amino acid sequences of the polypeptide chains. This allows accurate estimates of the number of water molecules associated with the protein molecule.
High-resolution X-ray diffraction studies on both native and synthetic collagens show an extensive water bridge network surrounding the collagen molecule. These and other structural studies have provided a molecular model of collagen with at least two categories of water. The most tightly bound consists of one highly immobilized water bridge for every three amino acid residues (0.0658 g water/g protein). A second less immobilized water fraction consists of three additional water molecules per tripeptide unit, residing in the three groove-like depressions between the peptide chains of the triple helix (0.197 g water /g protein). The tightly bound and three cleft waters together complete a chain of four water molecules per tripeptide, forming a triple helix of water in the three clefts between the protein chains that make up the triple helix. This implies a chain of water in each of the three grooves requiring a water content of 0.0658 + 0.197 = 0.263 g water/g protein.
Fullerton and colleague Maxwell Amurao first produced detailed calculations based on existing data and their own careful measurements on tendon diameter at different degrees of dehydration to convince themselves that the remaining water on native collagen is in the first monolayer covering the entire surface of the collagen molecule (1.315 water/g protein) [4], and not, as others have suggested, in multiple layers. This category of water is less immobilized than the first two categories, but still restricted in motion relative to bulk water.
They noted that fully hydrated bovine tendon in the native state has 1.62 g water/g protein. This is equivalent to 1.62/0.263 = 6.2 chains of water molecules per chain in the triple helix, or about 18 chains of water molecules per triple helix. This works out to be just sufficient to form a monolayer network over native tendon while maintaining the minimum spacing necessary to accommodate hexagonal water chains possessing the four hydrogen bonds per molecule as in bulk water. The arrangement of the 18 water chains around each triple-helix molecule is shown in Figure 1.
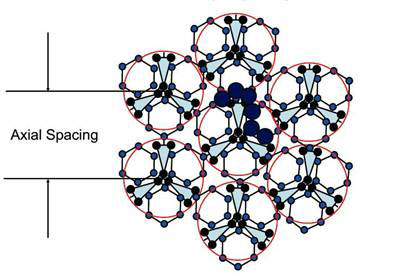
Figure 1. Cross section of a collagen fibril consisting of seven triple helices, each of which has 3 x 6 = 18 water chains covering it completely (modified from ref. 5)
Armed with this model, Fullerton and colleagues carried out measurements of nuclear magnetic resonance (NMR) relaxation times of water associated with native tendon at different dehydration levels.
The NMR ‘spin-lattice’ relaxation time is the time taken for the spin induced in magnetic nuclei (such as that of hydrogen) to relax back to equilibrium with its surroundings (the lattice), and is inversely correlated with how restricted its motions is. In other words, the more constrained its motion, the higher the rate of relaxation.
Fullerton and colleagues used a model in which all the categories of structured water exchanged protons rapidly even though they had different energy levels: the lowest being the most immobilized water molecules, and the highest the least immobilized, but still below that of bulk water. Using this model, they were able to identify categorical changes in water motion at critical hydrations levels (g water/g collagen) corresponding to 1, 4 and 24 water molecules per tripeptide unit of collagen, at 0.0658, 0.264, and 1.584g water/ g collagen respectively. This was precisely predicted by the model based on a monolayer of water covering the entire collagen molecule, and consists of 6 water chains in each cleft of the triple helix (see Figure 1).
The relaxation rates of the bulk water (reciprocal of relaxation time) was 0.347 s -1 for bulk water, 33.1 s-1 for the cleft water (the three water chains covering the deepest part of the clefts in the triple helix) and 1.351 s-1 for monolayer water (the remaining 15 water chains covering the rest of the collagen molecule (see Fig. 1).
In collaboration with Franco Musumeci and his team at the University of Catania earlier, we have investigated the delayed luminescence of bovine tendon at different stages of dehydration [8]. We found dramatic and abrupt changes in the delayed luminescence of bovine tendon at critical hydration levels, two of which are close to those identified here. Although our critical hydration values were less precise, the discontinuities we observed were more like phase-transitions. We identified four different phases, separated by rather sharp transitions at 1.52, 0.53 and 0.26 g water/g collagen. What could that possibly imply?
These observations imply that the structured water associated with collagen is highly ordered as a single phase at any time, and that at critical points of hydration, abrupt phase transition occurs to a different state of order, in analogy with ice structures. Finally, the fast exchange of protons in the structured water may also be an indication of proton jump-conduction extending throughout the structured water [9] (Positive Electricity Zaps Through Water Chains). The collagen water chains are reminiscent of those seen in carbon nanotubes (<5nm diameter) recently [10] (First Sighting of Structured Water), which are thought to represent an entirely new phase of water.
These are exciting times for biology, if only biologists would turn their eyes away for an instant from molecular nuts and bolts to more fundamental physics and chemistry.
Article first published 23/10/06
Got something to say about this page? Comment