How weak interactions can transform radioactive isotopes into more benign elements Lewis Larsen
The vast bulk of the world’s radioactive waste is created in uranium-based commercial fission reactors [1]. While some of that waste exists in the form of radioactive isotopes of gaseous elements and reactor components that have become radioactive from exposure to fast reactor fission neutrons, most nuclear waste is created and remains in reactor fuel rods [2] and related fuel assemblies where the raw nuclear heat for power generation is produced by nuclear fission reactions.
In spontaneous or neutron-triggered fission (in which an unstable fissile atomic nucleus absorbs a neutron), a heavy nucleus (e.g., uranium with atomic mass A = 235) violently splits apart into two ‘daughter’ nuclei; each fragment flying off with huge amounts of kinetic energy that creates intense heat when the fragments collide with surrounding materials in fuel rods [2, 3] (see Energy Strategies in Global Warming: Is Nuclear Energy the Answer? SiS 27). The fission process is asymmetric (the two daughter products almost always have unequal masses); also, it does not fragment exactly the same way every time, so a complex array of fission products with a broad range of many different masses is produced. While this fission product array includes virtually every element from zinc through the lanthanides, it is actually concentrated into two characteristic mass peaks: one from A = ~90 to 105 and a second from ~135 to 145 [4].
Unstable radioactive isotopes of the elements strontium (Sr), zirconium (Zr), technetium (Tc), and cesium (Cs) comprise perhaps the most abundant fission products produced in typical commercial reactors [4]. Other unstable fission products are also typically neutron-rich, and many (but not all) decay very rapidly via weak interaction beta processes (transmutation reactions) that may or may not be accompanied by gamma radiation emission. Different radioactive isotopes decay at different rates (half-lives), becoming stable, benign, non-radioactive isotopes over time. However, certain radioactive ‘hot’ isotopes with long half-lives remain biologically hazardous for many thousands of years.
In most present-day uranium-fueled fission reactors, roughly 25 percent of the U-235 originally present in the fuel rods when they were first loaded into the reactor still remains unburned when fuel rods reach the point at which they have accumulated enough ‘neutron poisons’ inside them that they cannot sustain a fission chain reaction. They are then considered ‘spent’ fuel rods.
In countries with ‘once through’ nuclear fuel cycle policies, spent fuel rods are simply removed from reactors, isolated in nearby ‘cooling ponds’ until their level of radioactivity decreases, and then ultimately shipped to a secure long term storage site (e.g., Yucca Mountain, Nevada, in the US). The ‘once through’ countries presently include the US, Canada, Sweden, Finland, Spain, and South Africa. The rest of the world uses some form of reprocessing of spent nuclear fuel in which “cooled” fuel rod assemblies are transported to strategically located reprocessing centers in which plutonium and uranium are separated from other materials and subsequently reintroduced into the nuclear fuel cycle. The remaining presently unusable isotopes from reprocessing spent fuel rods are then shipped to permanent nuclear waste storage facilities.
The whole issue of nuclear waste storage and reprocessing is highly controversial, raising serious questions on safety, sustainability, nuclear proliferation and economy [5] (see Nuclear Industry’s Financial and Safety Nightmare and other articles in the series, SiS 40)
Common elements and fission products/isotopes found in spent fuel rod assemblies from commercial fission power plants are presented in Table 1.
Table 1. Properties of material commonly found in spent fuel rods
| Materials Commonly Found In Spent Fuel Rods | Properties | ||||||
| Type | Element/Isotope | Half-Life (~ years) | Fission Yield ~ % | Normal Decay Mode | Thermal Neutron Capture Cross Section (barns) | Fission or Beta-decay Gammas? | Q-value for Beta Decay or Fission (MeV) |
| Fissile Fuels | Uranium U-233 | 159,000 | NA | alpha | 531 (fission) | Yes | ~190 (fission) |
| Uranium U-235 | 704 million | NA | alpha | 582 (fission) | Yes | ~190 (fission) | |
| Plutonium Pu-239 | 24,000 | NA | alpha | 752 (fission) | Yes | ~200 (fission) | |
| Fertile Fuels | Uranium U-238 | 4.5 billion | NA | alpha | 2.7 | No | NA |
| Thorium Th-232 | 14 billion | NA | alpha | 7.4 | No | NA | |
| Rod Cladding | Zr (5 isotopes) | NA - stable | NA | NA | 0.01 to 1.2 | NA | NA |
| Iron (5 isotopes) | NA - stable | NA | NA | 1.3 to 2.7 | NA | NA | |
| Long-lived Fission Products | Cesium Cs-135 | 2.3 million | 6.9 | Beta | 8.9 | No | .269 |
| Technetium Tc-99 | 21,000 | 6.1 | Beta | 23 | No | .294 | |
| Zirconium Zr-93 | 1.53 million | 5.5 | Beta | 2.7 | Yes | .091 | |
| Palladium Pd-107 | 6.5 million | 1.3 | Beta | 1.8 | No | .033 | |
| Iodine I-129 | 15.7 million | 0.8 | Beta | 20.7 | Yes | .194 | |
| Medium-lived Fission Products | Cesium Cs-137 | 30 | 6.1 | Beta | 0.25 | Yes | 1.2 |
| Strontium Sr-90 | 29 | 5.8 | Beta | 0.0097 | No | 2.8 | |
| Samarium Sm-151 | 90 | 0.5 | Beta | 15200 | No | .077 | |
| Krypton Kr-85 | 10.8 | 0.2 | Beta | 1.7 | Yes | .687 | |
Data compiled by Lattice Energy LLC; note that values found in different data sources are not entirely consistent with each other. The most worrisome items are highlighted in yellow.
From the standpoint of nuclear proliferation and radioactive waste, the most troublesome or hazardous materials commonly present in spent fuel rods include: U-233, U-235, Pu-239, Cs-135, Tc-99, Zr-93, Cs-137, and Sr-90. Radioactive cesium and strontium isotopes are particularly dangerous to vertebrates because, if they enter the food chain they can substitute chemically for calcium, thereby accumulating in calcium-rich bone material where they gradually decay, irradiating and damaging vital marrow cells. And this can severely depress the immune system.
‘Fertile’ isotopes such as U-238 and Th-232 can absorb neutrons without fissioning and, through a series of transmutation reactions, produce fissile Pu-239 and U-233 respectively.
A comparatively ‘slow’ 0.025 eV thermal-energy neutron moves at a speed of 2 200 metres/second [6]. By contrast, ‘fast’ 2 MeV neutrons produced in fission chain reactions travel at speeds a few percent of the speed of light. Regarding total neutron absorption cross sections (measured in “barns” - a barn is an area of 10-24 cm2), fissile materials such as U-233, U-235, and Pu-239 (along with many other, but not all, non-fissile isotopes) follow the low-energy region 1/v rule [7], v being the velocity of neutrons measured in metres per second. This means that the lower the velocity of an incident colliding neutron, the higher its absorption (capture) cross-section. Neutron absorption by 1/v isotopes is therefore much more efficient with slow neutrons than with fast ones; the slower the better. Importantly, ultra low momentum (ULM) neutrons created in certain low energy nuclear reactions (LENR) environments have kinetic energies that are vastly lower than those of thermal neutrons. Compared to speedy thermal neutrons, collectively created ULM neutrons are born almost ‘standing still’. This means that their capture cross-sections on 1/v isotopes will be vastly higher than those measured for neutrons at thermal energies.
Lattice has estimated the ULM neutron capture fission cross-section to be more than 1 000 000 barns for U-235, and >50 000 barns for Pu-239, compared to ~582 barns at thermal energies. By comparison, the stable isotope with the highest measured thermal neutron absorption cross section is gadolinium-157 at ~49 000 barns. Unstable Xe-135 (its half life is only ~ 9 hrs) has a measured thermal neutron capture cross-section of ~2.9 million barns. Given their unique absorptive properties, ULM neutrons could be used as extraordinarily effective tools for triggering fission in fissile isotopes and transmuting any isotopes that can capture extremely low-energy neutrons, i.e., follow the 1/v rule.
Weak interaction ULM neutrons have the potential to become a flexible technological tool that can be used to transmute one collection of target elements or isotopes into others; especially to clean-up radioactive wastes. For example, dangerous cesium, strontium, and technetium isotopes could be transmuted into stable elements [8] (Transmutation, The Alchemist Dream Come True, SiS 36).
LENR-based nuclear waste remediation techniques would entail a multi-step process of transforming entire spent fuel rod assemblies into specific types of nano-particulate targets with high surface-to-volume ratios that would enable them to come into close contact with locally generated LENR ULM neutrons. In principle, it could be a straightforward process that is technologically feasible and possibly very cost-effective.
Importantly, some aspects of a future LENR-based nuclear waste remediation technology have already been explored in the laboratory. Specifically, in a long series of important experiments, Dr. Yasuhiro Iwamura and his colleagues at Mitsubishi Heavy Industries in Japan have clearly demonstrated the transmutation of cesium to praseodymium and strontium to molybdenum by LENR ULM neutron-catalyzed reactions [9], consistent with the Widom-Larsen theory [10].
Similarly, the characteristic LENR ULM neutron transmutation product mass spectrum is probably known. We believe it was first discovered experimentally back in the mid-1990s by both George Miley [11] in the US and Tadahiko Mizuno [12] in Japan. Instead of the two-peak fission product mass spectrum obtained from present-day nuclear reactors, it is a distinctive 5-peak mass spectrum that appeared in Miley’s experimental data.
Working ‘backwards’ from the experimentally measured product spectrum, Miley interpreted this transmutation data as being a supposedly ‘slow’ fission spectrum of hypothetical unstable “complex nuclei” with atomic masses A = ~40, 76, 194, and one superheavy at A ~310, that were produced during the LENR process.
In our opinion, Miley’s interpretation of the above data was incorrect. On the contrary, according to the Widom-Larsen theory of LENRs, the data reflects a unique, characteristic signature of the absorption of large fluxes of ULM neutrons by atomic nuclei and related rapid beta decay processes. In that regard, we developed a simple 2-parameter optical model of ULM neutron absorption [13] that produces striking results when compared to Miley’s data (see Transmutation, The Alchemist Dream Come True, SiS 36 [7] for a simplified description of the model) .
The five peaks traced out by the solid line in Fig. 1 below [13] represent the output of the simple 2-parameter optical model of ULM neutron absorption that is simply overlaid on top of the product mass spectrum observed in one of Miley’s multiple LENR experiments. The five experimentally measured mass spectrum peaks in Miley’s data line-up with the model’s five calculated maximum resonance peaks for absorption of ULM neutrons as a function of atomic mass (A). The degree of correspondence is noteworthy.
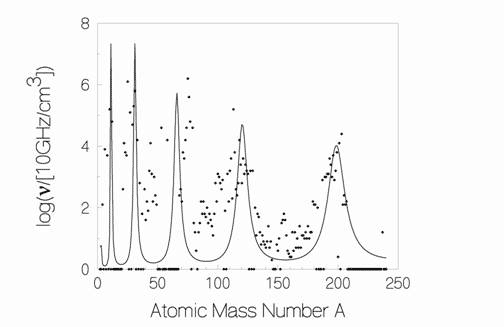
Figure 1. Miley’s experimentally observed isotopic production rates as a function of increasing atomic mass number is overlaid by the raw output of the Widom-Larsen theoretical ULM neutron optical absorption model with no forced fitting.
Importantly, Miley and Mizuno’s observed array of transmutation products did not contain any significant or detectible amounts of hot radioactive or fissile isotopes; nor hard gamma radiation and energetic neutrons. Such results are entirely consistent with the Widom-Larsen theory of LENRs [10]. This data also strongly suggests that absorption of large fluxes of LENR ULM neutrons by mixed isotopic systems likely produces very unstable, extremely neutron-rich intermediate nuclear reaction products that quickly transmute into stable isotopes via serial cascades of very rapid beta decays.
Consistent with Miley, Mizuno, and Iwamura et al’s experimental data [9, 11, 12], the Widom-Larsen theory of LENRs [10] implies that if you ‘cook’ a collection of different elements/isotopes long enough with appropriately large fluxes of LENR ULM neutrons, the resulting transmutation product spectrum will eventually contain a complex array of almost entirely stable isotopes. Over long ‘cooking times’, benign transmutation products should be distributed across 5 characteristic mass-peak regions (shown in Fig. 1 above) that would be very similar to what Miley and Mizuno discovered over a decade ago.
In the future, compact LENR ULM neutron generator systems could be developed and deployed for cost-effective on-site treatment of nuclear wastes presently stored in cooling ponds next to reactors that produced them. Spent fuel rod assemblies could be processed into particulates in on-site containment facilities and injected into co-located LENR-based transmutation reactors. These specialized reactors would then ‘burn’ hot radioactive wastes down to stable isotopes using large fluxes of ULM neutrons. If successfully developed, such a technology could significantly reduce nuclear waste remediation costs for decommissioning fission power plants, and significantly increasing their safety and profitability for those still operating.
Rather than just burning up spent fuel rod assemblies located at reactor sites or after removal of fissile isotopes at reprocessing facilities, excess heat generated during waste burn up with LENR ULM neutrons could be harvested with various types of power generation technologies to produce additional electricity that could either be utilized locally at a commercial power plant or connected and sold into the electricity grid.
There is also the potential to design and construct revolutionary subcritical ULM-neutron catalyzed fission reactors. That topic will be discussed in the final article of this series.
The author declares his commercial interest as President and CEO of Lattice Energy LLC.
Article first published 11/12/08
Got something to say about this page? Comment