All carbon micro-devices with large capacities, paper-thin or whisker-thick, print or spun that will accompany whole new generations of consumer microelectronics and wearable electronics; there is an urgent need to design for reuse and recycling now Dr Mae-Wan Ho
Supercapacitors - high capacity energy storage devices that deliver 10 to 100 times as much power as batteries and last 10 to 100 times longer – have been in use since the beginning of the present century, and on an exponential growth trajectory (see [1] Supercapacitors for Flexible Energy Storage & Ultrafast Superpower, SiS 66). However, there is still enormous room for improvement in materials and design to increase energy storage capacity and reduce weight/bulk and manufacturing cost.
Particularly promising are a range of all carbon micro-devices based on a combination of graphene and other recently discovered carbon allotropes (different structural forms of an element) such as carbon nanotubes. These are abundant, readily available and relatively easy to manufacture and shaped into high capacity electrodes. They are not subject to corrosion like metals and metal oxides, and hence longer lasting requiring little maintenance. They are also relatively non-toxic unless released or dispersed into the environment as carbon nanoparticles and/or especially as exotic electronic wastes.
There are two types of micro-supercapacitors: sheet-like ‘in-plane’ supercapacitors that can be laser-scribed or printed on flexible substrates to power displays; or directly onto the back of a chip for on-chip power, or on the back of solar panels, and other energy harvesting devices for energy storage. These can also be stacked up vertically, and/or rolled up into light-weight and compact larger supercapacitor arrays for stationary and mobile uses. We shall deal with these supercapacitors in Part 1.
The second type consists of thin fibres that can be integrated into microelectronics or woven into wearable electronics, incorporated into tents, screens, or artificial skin for robots, etc. These shall be the subject of Part 2 [2] (Graphene Liquid Crystal Electric Dream Coat, SiS 66).
It is still a challenge to compare the performance of different supercapacitors, as there is no generally agreed list of standardized tests to be carried out, or units in which the different measures of performance are to be reported. We begin with an example that gives the most complete characterization, which will be described in some detail; then move onto others that will be more briefly summarized.
Maher El-Kady and Richard Kaner at University of California Los Angeles used direct laser writing on graphite oxide films with a standard LightScribe DVD burner. We reported their results earlier (see [3] Graphene Micro-Supercapacitors for On-Chip Energy, SiS 59). They have now updated their findings that include the most complete set of tests [4], which will be instructive to follow in some detail.
Their most important finding is that miniaturizing the devices to the micron scale greatly enhanced charge-storage capacity and rate capability; resulting in a power density of ~200 W/cm3, among the highest values achieved for any supercapacitor.
The laser inside the DVD burner converts the golden brown graphene oxide into black laser-scribed graphene (LSG) on a flexible substrate into more than 100 potential micro-supercapacitor (MSG) units in ~30 minutes. Each printed unit under the microscope is seen to consist of a varying number of interdigitated electrode fingers (4, 8 and 16). Copper tape is applied along the edges to improve electrical contact, and the interdigitated area is defined by polymide (Kapton) tape. An electrolyte overcoat, a hydrogel polymer, poly(vinyl alcohol) PVA-H2SO4, is then added to create a planar, all solid-state LSG-MSG.
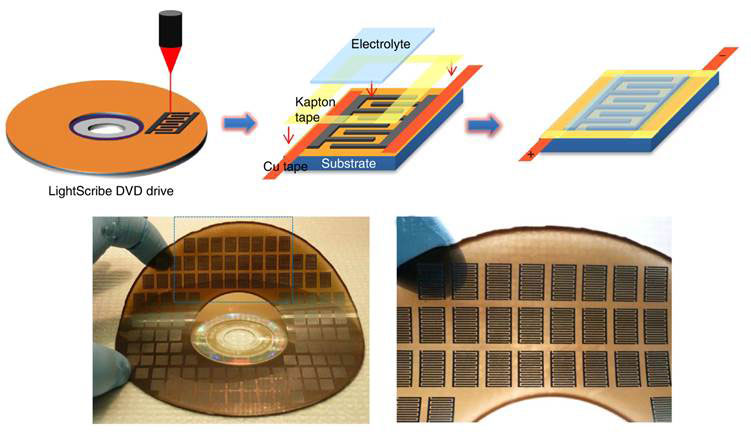
Figure 1 Fabricating graphene micro-supercapacitors on flexible substrates with DVD burner
The conductivity of the LSG is 2.35 x 103 S/m, six orders of magnitude enhanced over graphene oxide. On account of the high conductivity as well as exceptionally high specific surface area (> 1 500 m2/g), LSG can serve as both the electrode and current collector, thereby simplifying the fabrication process and saving costs. The thickness of the micro-device was 7.6 mm.
Micro-supercapacitors with 4, 8 and 16 interdigitated fingers - MSG4, MSG8 and MSG16 – were compared with a sandwich type LSG in which the electrolyte layer was sandwiched between two electrode/current collector layers of LSG.
First, they were compared on cyclic voltammetry (CV), a technique for measuring the current developing in the cell as the voltage is ramped up at linear (scan rates), then ramped back down to the initial potential. The current is plotted against the applied voltage to give the cyclic voltammogram. For an ideal capacitor (with zero electrical resistance) and fast response, the shape of the CV plot would be a perfect rectangle. The experimental plots are shown in Figure 2.
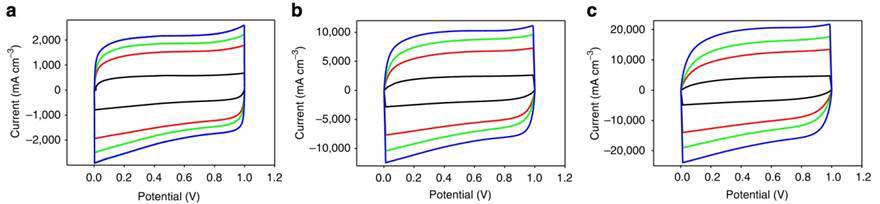
Figure 2 Cyclic voltammetry profiles of LSG-MSC sandwich (black), and interdigitated structures with 4 (red) 8 (green) and 16 (blue) fingers at scan rates of 1 000 mV/s (a) 5 000 mV/s (b) and 10 000 mV/s (c)
At scan rates of 1 000, 5 000 and 10 000 mVs-1, the current voltage curve remained roughly rectangular, indicating the high-power capacity of the microcapacitors.
They also investigated the specific volume capacitance of the micro-devices at different scan rates, including the combined volume of the active material, current collector and the gap between the electrodes as well as the electrodes. Micro-devices with interdigitated structure gave higher capacitance than the one with sandwich structure, and the more interdigitated electrodes per unit area, the more the power and energy that can be extracted from the device. The remarkable enhancement in performance with in-plane interdigitated electrodes is attributed to the much shorter diffusion path for ions, and maximising the surface area available for ion absorption.
The specific volume capacitance of the devices dropped with scan rates: that of the best performing LSG-MSC16 from 3.2 F/cm3 at 10mV/s scan rate to 1.7 F/cm3 at 10 000 mV/s, while that of the worst performing LSG-MSC sandwich decreased from 0.8 F/cm3 to 0.3/cm3.
Another important test is the cyclic charge discharge (CCD), a standard technique to test the performance and cycle life of supercapacitors. Most often, charge and discharge are conducted at constant current until a set voltage is reached. The charge (capacity) of each cycle is measured and the capacitance C in farad (F) is calculated. Both are plotted as a function of cycle number. Ideal behaviour is represented by perfect triangles (see Figure 4 later). CCD data are also plotted as a capacity curve, capacity vs cycle number. All the micro-devices, regardless of the number of interdigitated electrodes showed nearly ideal triangular charge/discharge curves at the ultrahigh current density of 1.85 x 104 mA/cm3, which is indicative of very low electrical resistance in the device (later estimated to be 3.6 W/cm2).
MSG16 has a volumetric capacitance of 3.05 F/cm3 at 16.8 mA/cm3, and maintained 60 % of the value up to an ultrahigh current density of 1. 84 x 104 mA/cm3. That is equivalent to operating the device at ~1 100 A g-1 LSG/electrode, three orders of magnitude higher than the normal discharge current densities used for testing traditional supercapacitors. It corresponds to an area capacitance that varies only from 2.32 mF/cm2 at 16.8 mA/cm3 to 1.35mF/cm2 at 1.84 x 104 mA/cm3.
The micro-supercapacitors showed superior frequency response with an extremely small relaxation (recovery) time. An important quality measure is the RC (resistance capacitance) time constant (in seconds), equal to the product of the circuit resistance (in ohms) and the circuit capacitance (in farads), and is the time required to charge or discharge the capacitor, through the resistor by ≈ 63.2 % of the difference between the initial value and final value. The RC time constant of MSG16 was 19 ms, compared with 10 s for the commercial carbon supercapacitor, and 1.1 ms for an aluminum electrolytic capacitor. It is also comparable to previously reported values for planar micro-supercapacitors of activated carbon, 700 ms; onion-like carbon, 26 ms; and graphene/carbon nanotube composite, 4.8 ms.
The LSG-MSGs showed excellent cycling stability, retaining 96 % of the initial performance after 10 000 charge/discharge cycles.
The LSG-MSGs were tested under conditions of twisting and bending (Figure 3a). The CV profiles were unchanged at scan rate of 1 000 m V/s (Fig.3b). The specific capacitance was also reversibly maintained with 97% retention of initial capacitance after 2 000 cycles of bending (Fig. 3c).
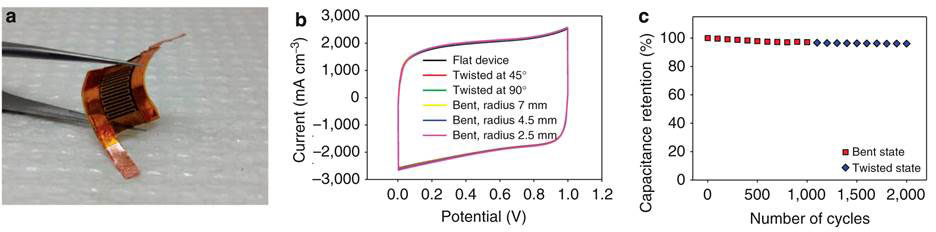
Figure 3 Performance of LSG-MSC on twisting and bending (see text for details)
In general, the total energy that can be stored in a single supercapacitor is too small for most practical applications. So, the devices need to be connected together in series or in parallel, just as batteries are, to form a bank with a specific voltage and capacitance rating.
How do the micro-devices behave in tandem? El-Kady and Kaner tested that too. The tandem of 4 LSG-MSC exhibits a very good control over the operating voltage window and capacity, thus enabling them to be considered for practical applications. Similar to individual LSG-MSC, the tandem devices exhibit essential ideal triangular charge/discharge curves with a minute voltage drop (Figure 4). This excellent performance has been achieved without using a voltage balance, which is often needed with series connections to prevent any cell from going into over-voltage.
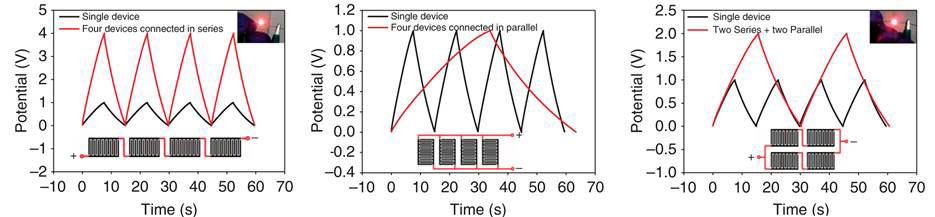
Figure 4 Charge/discharge curves of four devices variously connected in tandem
Finally, the micro-devices can be directly laser-scribed onto a chip that contains integrated circuits, which they can then power. For this application, El-Kady and Kaner used a different electrolyte, the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide with fumed silica nanopowder to form a viscous ionogel (FS-IL). The completely solid-state device exhibited the same high rates of charge/discharge as PVA-H2SO4, but operated at a large potential window of 2.5 V instead of 1 V.
A Ragone plot of energy density versus power density is presented for the different micro-devices fabricated by El-Kady and Kaner in comparison with the commercially available lithium thin-film battery, an activated carbon supercapacitor (AC-SC) and an aluminum electrolytic capacitor (Figure 5).
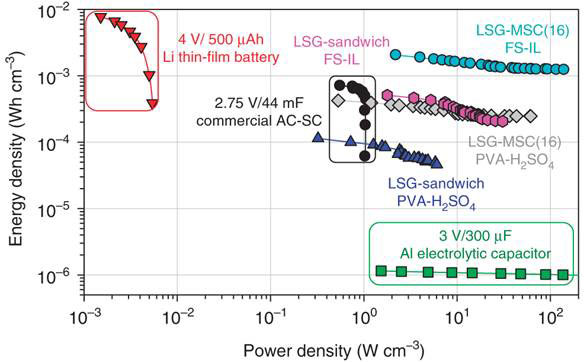
Figure 5 Ragone plot of energy density vs power density of different energy storage devices (see text for details)
As can be seen, the FS-IL has the best overall performance in comparison with LSG-MSC with Polyvinyl alcohol (PVA-H2SO4) electrolyte, as well as commercially available lithium thin-film battery, activated carbon supercapacitor (AC-SC) and an aluminum (Al) electrolytic capacitor. It reveals a significant increase in the supercapacitor performance in scaling down the electrode to micron dimension. The interdigitated micro-supercapacitors deliver more energy and power than their sandwich counterparts both in the hydrogel-polymer and ionogel electrolytes. Compared with the AC supercapacitor, the LSG micro-device delivers 3 times more energy and ~200 time more power densities. The LSG-MSC demonstrates power densities comparable to those of the aluminum electrolytic capacitor, while providing more than 3 orders of magnitude higher energy density. Although Li-ion batteries can provide high energy density, they have limited power performance 4 orders of magnitude below those of LSG-MSC. This superior energy and power performance of LSG-MSC makes them competitive with micro-batteries and electrolytic capacitors in a variety of applications. Further miniaturization is possible with LSG-MSC, which could lead to even higher energy and power densities.
The solid state enables the micro-supercapacitors to be more easily shaped for incorporating into any new device, or they could be fabricated directly onto a chip for integration into micro-electromechanical systems (MEMS) and complementary metal-oxide-semiconductors (CMOS). This may help to better harvest energy from solar, mechanical and thermal sources and make more efficient self-powered systems. The solid-state micro-supercapacitors could be fabricated on the backside of solar cells in both portable devices and rooftop installations to store power generated during the day for use after sundown, and provide electricity, potentially around the clock without connecting to the grid.
One potential area of improvement not mentioned by the researchers is to laser-write customized tandem arrays to minimize external connectors, thereby decreasing cost and bulk. We shall look at other examples briefly in comparison.
A research team at Rice University, Houston, Texas in the United States used laser induction to create porous graphene directly on commercial polyimide films to make flexible, solid-state suspercapacitors [5]. The devices with a solid state polymeric electrolyte exhibited area capacitance of >9 mF/cm2 at a current density of 0.02 mA/cm2. Laser induction on both sides of the polyimide film enables the fabrication of vertically stacked supercapacitors that can then be connected in series or in parallel. The supercapacitors were unaffected by bending, and stable to charging and discharging, as well as bending for thousands of cycles.
This invention has the advantage that it is very easy to make and scale up. But how does the capacities of the device compare to the LIG-MSC made by El-Kady and Kaner [3]? They reported an area capacitance of 2.32 mF/cm2 at 16.8 mA/cm3, a smaller capacitance but at much greater current density, bearing in mind that capacitance generally decreases with current density. Also, the LIG-MSCs were 7.6 mm thick; whereas the single cell fabricated by the Rice University team was >100 mm thick. Consequently, they have energy density at best half of those of LIG-MSCs, and power densities 2 orders of magnitude smaller.
Researchers Wu Zhong-Shui, Kaled Parvex, Feng Xinliang and Klaus Müllen at Max Planck Institute for Polymer Research, Mainz, Germany fabricated a graphene-based in-plane interdigital microsupercapacitors on both rigid and flexible substrates [6]. The resulting microdevices with graphene films ≤ 100 nm thick give an area capacitance of 80.7 mF/cm2 and a stack capacitance of 17.9 F/cm3. They show a power density of 495 W/cm3, higher than electrolytic capacitors, and an energy density of 2.5 mWh/cm3, comparable to lithium thin—film batteries, and a superior cycling stability. The RC time constant was 0.28 mS. Such microdevices allow for operations at ultra-high rate up to 1 000 V s-1, three orders of magnitude higher than conventional supercapacitors. The supercapacitors also show exceptional stability, retaining 98.3 % of its capacitance after 100 000 cycles at scan rate of 50 V/s.
Exactly the same design was used on a flexible substrate of polyethylene terephthalate (PET). The performance of the device was almost identical, retaining 99.1 % of capacitance after 100 000 cycles at a scan rate of 200 V/s and no capacity degradation after the device was bent 100 times.
The key to the superior capacitance of the device is the graphene film manufactured by spin-coating a silicon wafer (pretreated with oxygen plasma) with a graphene oxide dispersion, then rapidly reducing the film with methane-plasma at 700 C. However, the manufacturing process is elaborate, and a gold collector is used, all of which increases cost.
The supercapacitors described so far all involve graphene as major capacitance material (electrodes or electrodes and collectors); however, they still include the use of metal as collector or to improve contact.
Researchers at Queensland University of Technology Brisbane, Australia and Rice University Houston, Texas, have fabricated genuinely all carbon high performing supercapacitors using graphene in combination with carbon nanotubes [7, 8].
In the first reported version [7], a combination of a 360 nm thick graphene film and carbon nanotube (CNT) as electrodes resulted in a capacitance of 4.3 mF/cm2, whereas the CNT alone gave a capacitance as low as 0.4 mF/cm2. However, the conductive CNG film is equivalent to gold as a current collector while providing a stronger binding force to the graphene film. The resulting devices gave a high energy density of 8-14 Wh/kg and power density of 250-450 kW/kg.
The CNT films were prepared from commercially available double walled carbon nanotubes (DWCNT) with an average length of 10 mm and average diameter of 2.4 nm., dispersed in solvent and vacuum filtered through alumina membranes dried, the alumina membrane etched away, washed, and transferred onto a polyethylene naphthalate (PEN) paper, then dried. The CNT film was 520 nm thick (weighing 29 mg/cm2) with a sheet resistance of 5 W/sq. The graphene was prepared by electrochemically exfoliating highly oriented pyrolytic graphite (1 x1 cm2) in aqueous solution, then vacuum filtered through alumina membrane to form a uniform film, dried, the alumina filter etched away, the resulting graphene film further washed, transferred to PEN and dried. For comparison, a graphene film was also transferred onto a Au-coated PEN paper for making a device.
Three devices were fabricated, the first (D1) with 520 nm CNT film only, the second with 520 nm CNT film and a 85 nm layer of graphene (D2), and the third (D3)with a 85 nm layer of graphene and gold as collector. D2 and D3 showed almost identical capacitances much higher than that of D1. D2 was tested by repeated charging and discharging at 25 mA/cm2 for >3 500 cycles, and found to retain 89 % of initial capacitance.
Increasing the thickness of the graphene layer increases the capacitance, but the mass specific capacitance decreases. A 360 nm thick layer of graphene resulted in an area capacitance of 4.3 mF/cm2.
In the second report [8], multiwall carbon nanotubes (MWCNT) were mixed with graphene in a composite film to serve as electrode, while DWCNT served as collector. It resulted in a volume energy density of 1 mWh/cm3, and a power density ~ 10W/cm3.
A team of researchers led by Yongsheng Chen at Nankai University Tianjin, China, have succeeded in making a 3-dimensional graphene-based porous material in bulk with an ultrahigh specific surface area (SSA) of 3 523 m3/g and high conductivity (up to 303 S/m), which consists of mainly defective/wrinkled single layer graphene sheets several nanometres in size covalently bonded together. They give a specific capacitance of 231 F/g and energy density of 98Wh/kg. The process is simple. It involves a hydrothermal polymerization/carbonization of a mixture of cheap biomass or industrial carbon source with graphene oxide (GO) to get a 3-D precursor, which is then chemically activated to yield a material with the desired SSA and conductivity. The yield of carbon atoms in the first step is almost 100 %, a significant improvement on the usual industrial process also in terms of financial and environmental costs. Both steps are industrially scalable. The pore sizes are mainly in the mesopore range, which is responsible for their superior performance as supercapacitors.
The industrial carbon materials include sucrose, cellulose, lignin, polyvinyl alcohol, phenol and formaldehyde. These are mixed with small amounts of GO. For example, the optimum mixture of polyvinyl alcohol with GO is 20:1 by weight, resulting in a product PVA20 GHA with SSA of 3 192 m2/g and conductivity of 67 S/m; while the optimum mixture of phenol and formaldehyde with GO is 16:1, resulting in the product PF16GHA with SSA of 3 523 m2/g and conductivity of 303 S/m.
As supercapacitor electrodes with EMIMBF4 (1-ethyl-3-methylimidazolium tetrafluoroborate) as electrolyte, PVA20GHA gives a specific capacitance of 231 F/g, an energy density of 98 Wh/kg and power density of 137 kW/kg. The corresponding values for PVA20GHA are 207 F/g, 88 Wh/kg and 87 kW/kg.
These supercapacitors are stable to charge/discharge cycles. PF16GHA for example, retained 94 % or more of its specific capacitance in a range of electrolytes after 5 000 cycles.
The team also constructed high-voltage internal tandem supercapacitors using the similar graphene-based porous material made from lignin and GO at a ratio of 50:1. Compared with the packaged energy density of 27.2 Wh/kg and working voltage of 3.5 V using electrolyte EMIMBF4 for the usual single cell supercapacitor, the internal tandem device achieves a working voltage of 7 V and a significantly improved energy density of 36.3 Wh/kg (representing an enhancement of 33 %) (see Figure 6). This is about 7 times that of the state of the art commercial supercapacitors. It retained 89 % of initial capacitance after 3 000 cycles at 2A/g. The internal tandem device reached an energy density of 36.3 Wh/kg and a power density of 305.7 W/kg. A flexible internal tandem device was also made, which demonstrated similar outstanding performance. It withstood severe bending up to a completely folded condition in cyclic voltammetry tests as well as cyclic charge/discharge.
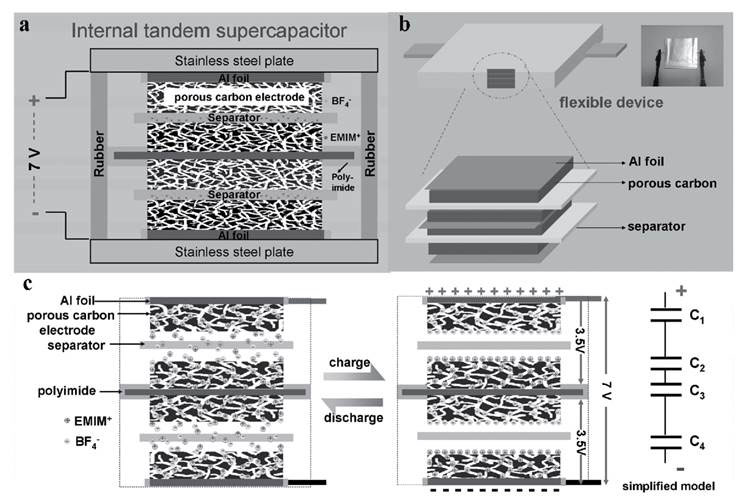
Figure 6 Internal tandem supercapacitor, rigid version between stainless steel plates (a), flexible version packaged in flexible aluminum foil (b), separation of charged ions in the electrolyte to BF4- and EMIM+ creating serial electric double layers on the surfaces of the electrodes of the tandem device when charged up and diffusing back into solution when discharged (c)
Article first published 01/04/15
Comments are now closed for this article
There are 1 comments on this article.
Todd Millions Comment left 2nd April 2015 17:05:02
The waste carbons mixed with the graphene -a report last year had controlled charring of hemp waste(from fibre production) producing graphene fragments thought to be suitable for capacitors.Save the Roaches!(wry). Since capacitors are also crucial in soaking up stray voltage transients(still the limit condition on many buzzy circuits of what ever scale)-Could these thin film designs be used as power sources and a substitute for the Coaltan shielding required too keep computer and radio equipment from melting down?Having the power storage on the inside of the device shell-also provide the emf shielding?This could save on cost and slow the looting of the Congo.