Post-mortem brains from ALS patients show increased expression of endogenous retroviruses which when introduced in mice, lead to neurodegeneration Dr Eva Sirinathsinghji
A new study published in Translation Medicine Online [1] links risks of developing amyotrophic lateral sclerosis (ALS) to the activation of endogenous retroviruses (ERVs) present in the human genome, a type of mobile genetic element thought to be remnants of ancient retroviral infections. Until recently, ERVs were commonly presumed to form much of the so-called redundant ‘junk’ DNA, but they are being revealed to have important physiological function.
The idea that these mobile elements are acting ‘selfishly’ has lost ground to the evidence showing them to be symbiotic partners that integrated into the human genome millions of years ago. Abnormal activity of endogenous retroviruses is now being linked to many diseases including ALS, cancers, autoimmune conditions such as arthritis psoriasis and lupus, schizophrenia, neurological dysfunction, diabetes and HIV.
ALS (aka motor disease, Lou Gehrig's disease and Charcot disease) is characterised by progressive degeneration of the motor neurones, leading to muscle wasting and eventual death due to respiratory muscle failure. The cause of the disease is not yet understood, though a small proportion of cases (5-10 %) are due to genetic mutations.
Viral infections have been suspected to play a role in ALS since the 1970s when evidence of retroviral expression was noted in ALS patient brains, and have been proposed to come from either exogenous viruses or, as shown in this new study, from the activation of endogenous retroviruses present in the human genome.
The study led by Avindra Nath at US National Institutes of Health decided to probe further into his previous observation that treatment of one of his HIV-positive patients who also suffered ALS with antiretroviral drugs, resulted in the reversal of ALS symptoms [2]. Reverse transcriptase, an enzyme mainly associated with retroviruses that functions to transcribe (copies) DNA from RNA (the process that defines a retrovirus), has also been previously found in ALS patient blood without an accompanying presence of exogenous retroviruses [3-7].
Looking into the brains of 16 post-mortem ALS patients and 11 controls without evidence of neurodegeneration, the researchers found high expression of human endogenous retroviruses (HERVs). One family in particular called HERV-K was more highly expressed in large pyramidal neuronal cells of the cortex, as well as the neurones in the anterior horn of the spinal cord, regions that are particularly affected in ALS (see figure 1). There was no upregulation of HERV-K expression in brains from patients with other neurological disease.
To test the functional role of upregulated HERV-K expression in human ALS brains, the researchers transfected human neuronal cell cultures with HERV-K. Neurones expressing HERV-K showed signs of pathology including dose-dependent decreases in cell numbers as well as neurite length (projections from the cell body of a neurone). They found similar results when they activated the endogenous HERV-K in neuronal cell cultures by introducing a transgenic promoter to drive its expression using the CRISPR/Cas9 gene editing technique.
Similarly, in mice, introducing the HERV-K gene in utero into the brains of embryos resulted in reduced number, length and complexity of dendrites (neuronal projections): reduced dendritic mushroom spines, as well as increases in dendritic stubby spines (stubby and mushroom spines being different classes of dendrite spine shape). Transgenic animals expressing HERV-K also showed ALS-specific degeneration of neurones, with no reduction in overall neuronal numbers but a decrease in Ctip2-positive cells. Ctip2 is used as a marker for corticospinal motor neurones. They had a near absence of motor neurones in the anterior horn of the spinal cord and significant reduction (22 %) in the thickness of their motor cortex, but no difference in other brain regions such as the hippocampus, corpus callosum or cingulate cortex. The mice showed increased neuronal injury as measured by an increase in DNA damage. Behavioural changes matched ALS symptoms, the affected mice showing a progressive decline in motor function from 3-6 months of age and a 50 % mortality rate at 10 months of age.
Looking into the mechanism of upregulated HERV-K expression, the researchers found that TAR- DNA-binding protein 43 (TDP-43) controls HERV-K expression when transfected into neuronal cultures. TDP-43 has long been implicated in ALS and is known to regulate retroviruses such as HIV, and also binds to transposable elements. Its pattern of expression has previously been shown to correlate with that of HERV-K in ALS patient post mortem brains. Over 30 mutations in this protein have already been linked with ALS and another neurodegenerative disease frontotemporal lobar dementia (FTLD), which has also been found in the protein aggregates accumulate in the brains of ALS patients, and is a pathological hallmark of ALS and other neurodegenerative diseases. Even overexpression of wild-type TDP-43 is sufficient to induce motor neurone degeneration in mice. The activation of HERV-K by TDP-43 therefore provides a new mechanistic link between TDP-43 and ALS.
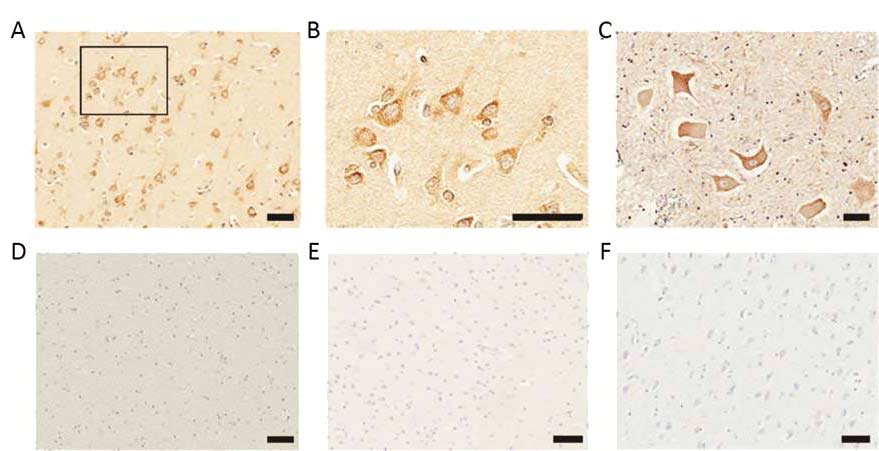
Figure 1 Elevated expression of the human endogenous retrovirus in neurones of post mortem brains from ALS patients; (A) Representative image of Immunostaining of HERV-K in ALS patient brains (B) A higher magnification image of the boxed region in A, showing focal accumulation of HERV-K at the cell membranes of cortical neurones (C) Immunostaining o HERV-K in anterior horn neurones. There was no positive immunostaining in (D) the white matter region of the ALS brain, (E) a normal brain of a motor vehicle accident or (F) or a brain from an Alzheimer’s disease patient
Scale bars, 50 mm
It is estimated that these small viruses were first integrated into the human genome 30-40 million years ago, and are now transmitted down the generations in Mendelian fashion and found within the genome of all cells. There are different types of retro-elements in the human genome in addition to ERVS, termed LINES (long interspersed elements) and SINEs (short interspersed elements) that have also been shown to have important physiological functions. HERVs constitute an estimated 8 % of the human genome, and if including HERV fragments and derivatives, the retroviral legacy amounts to roughly half of our DNA [2, 3]. Their genomic structure is similar to exogenous retroviruses, and is composed of three genes gag, pol and env between two long terminal repeat regions (LTRs) (see Figure 2). LTRs are identical sequences repeated hundreds or thousands of times and are used to insert their genetic material into the host genomes. They are also the control centre for gene expression, carrying all the necessary regulatory components for gene expression. The Gag proteins are major components of the viral capsid, while Pol proteins are responsible for synthesis of the viral DNA and integration into host genome, and Env proteins play a role in association and entry of viral particles into the host cell. Most HERVs have become defective, unable to replicate due to mutations in their genetics. HERV-K is however able to generate viral particles. There are over 26 families of HERVs already identified, and although most have become defective due to mutations and deletions in their sequences, the HERV-K family produce intact viral particles.

Figure 2 Genomic structure of human endogenous retroviruses
HERVs, of all the mobile elements, are of particular interest due to their regulatory components located within the LTR regions. The regulatory elements within them can induce and mediate expression of the viral genes as well as expression of nearby human host genes. Such regulatory elements include gene promoters, enhancers, polyadenylation signals, insulators as well as binding sites for various nuclear proteins including transcription factors. HERV genes also have the potential to integrate in any location in the genome, therefore altering genomic structure. It has been suggested that they provide sequences that allow for genomic alterations in what has been described as ‘natural gene engineering’ (see review [9]). They are a source of novel genetic coding and non-coding information used by non-coding RNAs, splice signals for genomic rearrangements as well as elements important for epigenetic control of the genome. Indeed, recent bioinformatics research has identified around 110 000 regulatory active HERV elements around 320 000 human transcriptional binding sites [10].
Evidence of their functionality come from studies in humans showing that 30 % of healthy individuals are expressing HERV genes, while increased production has been found in placentas and embryonic tissues, in line with the identification of hormone response elements in their regulatory regions. Indeed, HERVs have co-evolved with the human genome and play important molecular functions including the development of the mammalian placenta through the expression of the syncytin protein from the HERV-W env gene involved in the fusion of placental cytotrophoblast cells to form one of the placental layers called the syncytial layer, which is vital for embryo survival (see review [11].
One example of their role as transcriptional regulators comes from studies of the hippocampus – the brain region central to learning and memory. Enhancer elements (involved in transcriptional activation of genes), were found to mediate expression of a hippocampal PRODH gene, created through the insertion of an LTR from HERV-K. Abnormal expression of HERVs has also been linked to schizophrenia, depression and bipolar disorder, with brains of patients showing reduced expression of HERV-W when compared with healthy brains, suggesting a loss of function as opposed to over-activation of the retroviruses in this scenario [12]. Another HERV family, HERV-H, preferentially expressed in stem cells, was recently shown to mediate stem cell fate programming through the HERV-H driven non-coding RNA transcripts [13]. Other forms of endogenous retro-elements such as short interspersed elements (SINEs) have also been implicated in brain development [14].
While some HERVs such as the HERV-K family appear to be expressed under healthy conditions, most are silenced through mutation or tightly epigenetically controlled via methylation of DNA or chromatin modifications. However, studies have found that environmental stimuli can induce abnormal activation linked to pathological conditions. Examples of this include microbial activation as well as activation via acute viral infections such as HIV, Epstein-Barr virus, herpes virus 6 and human cytomegalovirus. Indeed, a recent Nature study found that mice that are immunocompromised showed activation of mouse ERVs, but this activation did not happen when mice were placed in germ-free conditions associated with reduced or absent microbiota [15]. This seems to suggest an intricate symbiotic relationship between immunity and the control of ERVs, providing a mechanistic link between immune activation and pathologies.
Other factors linked to HERV activation include pro-inflammatory cytokines suck as interleukin 1β, tumour necrosis factor α and interferon α, the latter shown to induce expression of HERV-K superantigens. Superantigens are microbial products that have the ability to promote a massive immune response implicated in autoimmunity. Viral infections have long been associated with autoimmunity and endogenous retroviruses therefore present another possible underlying mechanism to many autoimmune diseases where evidence of exogenous infections is lacking.
Organophosphate pesticides, steroid hormones, DNA mutagens such as UVB radiation, bacterial infections and retinoic acid have all been shown to activate HERV expression (see [16] Endogenous Retroviruses and Chronic Disease, SiS 19). Other factors such as hypoxia have been shown to reduce expression of HERV proteins.
Additional environmental factors linked to ALS include smoking, chemical exposure in many occupations, with farm workers, rubber workers, leather workers being overly affected; and metal exposure (lead, iron and selenium are often increased in ALS patients, as well as in medical conditions such as cancers, neuro-inflammation and head trauma) [17]. It would be of interest to investigate further whether any of these risk factors are associated with inducing abnormal HERV activity. As mentioned above, activation of HERVs via chemical exposure in a farm setting may be due to organophosphate exposure, while endocrine disrupters could also be a factor, with HERVs having hormone-response elements within their genomes. Neuroinflammation, infections with numerous pathogens, as well as dysbiosis of the microbiome may all be having an influence on HERV activity.
Activation of endogenous retroviruses that are abundant within the human genome are intricately connected to human evolution and add another layer of complexity to the symbiotic relationship between our bodies, environment and ancient viruses that have become vital to human physiology and survival.
Article first published 14/12/15
Got something to say about this page? Comment
There are 2 comments on this article so far. Add your comment above.
Rory Short Comment left 15th December 2015 06:06:12
Very interesting. As a sufferer from relapsing remitting Multiple Sclerosis I have noticed that infections such as tooth decay and 'flu give me MS Relapses. My work,on the psycho-emotional problems laid down in my childhood, seems to have eliminated any MS relapses, that prior to the inner work, would have been provoked by psycho-emotional events in my current life. I am not a micro-biologist but it has always seemed obvious to me that there is a connection through infections between my immune system and my MS relapses.
Todd Millions Comment left 23rd December 2015 18:06:11
Thank you for this- Keeping up with these developments would be near impossible without this site. I live in an area where MS in particular is inexplicably high. This doesn't seem too be the case till after WW2,but statistics are strangely absent too indicate that.The upswing was when several things like atmospheric atomic bomb testing,and OP insecticides were-introduced according to my grandmother.
Herbal/ vitamin combinations for viral infections have being effective in my personal use. But the herb I use is now formulated differently by statute and is no longer available in a efficacious form. I had best work on better supply.The synthetic patented versions of it are rated almost as effective and not much more toxic. I would like to see if the original works on retro viral infections-first.