Solar power is by far the most abundant renewable zero-carbon energy resource, and artificial photosynthesis could be the most effective way to store the energy and make it more available and affordable. Dr. Mae-Wan Ho
Global energy demand is projected to increase by 57 percent from 14.9 TW (1 TeraWatt = 1012W) in 2004 to 23.4 TW in 2030 [1]. There is considerable urgency in developing ‘carbon neutral’ power if CO2 level is to be kept below the 550 ppm target set by the Intergovernment Panel on Climate Change that most governments accept. Three main routes are being considered: nuclear fission, carbon capture and storage, and renewable energies.
Apart from being inherently unsustainable as well as unsafe and uneconomic [2] (see The Nuclear Black Hole and other articles in the series, SiS 40), the estimated uranium resources remaining are only sufficient to produce ~100 TW-yr of electricity and would be exhausted within a decade. Carbon capture and storage (CCS) in underground aquifers, likewise, is too late to be of use and much too expensive and ineffective [3] (Carbon Capture and Storage A False Solution, SiS 39). A study commissioned by the German federal government found that CCS emits ten to forty times as much greenhouse gases as wind or solar energy, and gives no protection against the rising costs of fossil fuels [4] (Renewables versus Carbon Capture and Storage, SiS 39). To be viable, the carbon dioxide captured and stored must leak at a globally averaged rate of not more than one percent over a timescale of centuries; otherwise, the emitted flux will be greater than or equal to that intended to be mitigated initially [1]
Among renewable energies, by far the largest resource is provided by the sun [1]. Solar energy reaches the surface of the earth at the enormous rate of ~ 120 000 TW; but only a minute fraction, <0.001 percent, is currently harnessed for use to produce the 145 GW current global capacity [5] (see Global Shift to Renewable Energies Happening, SiS 43).
There are numerous ways to harvest sunlight, which involves capture and conversion; but storing the energy is a problem.
Solar capture and conversion is accomplished by existing photovoltaics (PVs), which turn sunlight into electricity, and especially solar thermal, which captures sunlight to heat (and cool) water and spaces. Concentrating solar power using focussing mirrors that track the movement of the sun throughout the day is the extreme end of solar thermal, and is capable of producing heating power equivalent to thousands of suns [6]. Heat generation is also being combined with electricity generation in the same module. Combined heat and power solar are now produced in Europe, and being developed in Australia, America and China.
However, the sun shines intermittently, and then only during the day. So it is necessary to have efficient and cost-effective storage capacity, if solar is going to become a primary energy source for society [1]. Solar power already leads in the renewable energies market [5], and as the world is shifting to renewable over conventional fossil energies, we should aim for an integration of capture, conversion, and storage functions for solar power.
In principle, electricity can be stored in batteries, but batteries are still too costly. Another method is to store the electrical energy mechanically by using it to pump water uphill; but this will mean charging and discharging on a 24 h diurnal cycle. For buffering the day/night cycle in the US energy demand, this would require the pumping capacity equivalent to more than 5 000 Hoover Dams filling and emptying reservoirs every day and night. In solar thermal, energy can be stored in water in an insulated thermal reservoir above or below ambient temperatures, which can then be used to heat spaces during the night or cool spaces during the day [7].
A method for storing solar energy has already been invented by nature, and that is photosynthesis, which uses sunlight to split water, releasing oxygen, and fixing carbon dioxide into carbohydrates with the hydrogen, and creating biomass [8] (see Living with Oxygen, SiS 43). Photosynthesis has effectively provided the world with food, fibre, building material, and fuel (in biomass and fossil energies). The recent boom (and bust) of ‘bioenergy’ crops to supply ‘biofuels’ has been disastrous in accelerating deforestation and pushing up food prices especially in the developing world [9] (Biofuels: Biodevastation, Hunger & False Carbon Credits, SiS 33).
The problem with photosynthesis, as far as capturing sunlight for other uses is concerned, is that it that it has not evolved to maximise efficiency in harvesting solar energy, because solar energy is rarely limiting; and there are many mechanisms plants have evolved to protect themselves from oxidative damages that strong sunlight can inflict.
It is estimated that the theoretical maximum efficiency of photosynthesis is ~9 percent [10]. This instantaneous efficiency would only be achievable under low light intensity, where every incident photon of appropriate wavelength can be absorbed and used for productive electron transfers (see below). Under full sunlight, natural photosynthesis uses only a fraction of incidence photons. Downstream carbon fixation further reduces the attainable efficiency; and many photosynthetic organisms have seasonal variations in photosynthetic rates. Consequently, on an annual basis, photosynthetic efficiencies average at best < 0.2 percent for land bioenergy crops and < 5 percent for microalgae [11] (but see [12] Saline Agriculture to Feed and Fuel the World, SiS 42).
One approach to storing solar energy is artificial photosynthesis, which attempts to replicate and improve on the natural process, mainly to obtain hydrogen as fuel for use in fuel cells, and includes the photoelectrochemical splitting of water into hydrogen and oxygen (the inverse of a fuel cell, where hydrogen and oxygen recombine to give water, releasing the energy stored in hydrogen) (see Fig. 1) [13]. In a photochemical or photoelectrochemical (PEC) system, a photoactive semiconductor material forms a junction in contact with a liquid or solid electrolyte. Because of the junction potential, electron-hole pairs are produced in the photoactive material on illumination. The light-induced electron-hole pairs (e-and H+ in the case of water) drive a chemical reduction (left, Fig. 1) and oxidation (right, Fig. 1)] leading to hydrogen and oxygen evolution respectively. Water is thereby split into its elements in two half-reactions, oxidation of water to oxygen, and reduction of protons to hydrogen, each of which requires its own catalyst and optimised conditions. In this way, the photon energy is converted directly into chemical energy rather than into electrical energy as with solid-state or electrochemical PV cells.
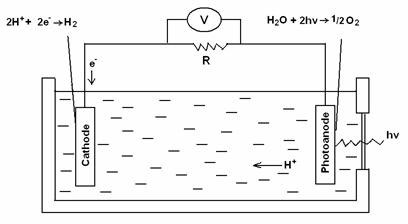
Figure 1.The photoelectrochemical cell
The fundamental requirement for the conversion of sunlight into fuel is the oxidation of (removal of electrons from) a low energy electron source to produce a high energy reduced chemical species (that accepts electrons) [14]. In photosynthesis of green plants, water is the ultimate electron donor. Water is an ideal source of electrons because of its low energy content, abundance, and the production of O2 which can be allowed to react on demand with the reduced fuel, H2, for releasing energy.
The inter-conversion between oxygen and water is described by eq (1), where hn represents a photon of the appropriate wavelength for photosynthesis (see also Fig. 1).
O2 + 4hn <-> 2H2O (1)
In photosynthesis, the electrons extracted from water are boosted in energy by sunlight, so it can produce the high energy reduced chemical species. From a thermodynamic perspective, the production of hydrogen (reduced proton) is approximately equivalent to the reduction of coenzyme NADP+ and ultimately, CO2 to carbohydrates.
4H+ + 4e- <-> 2H2 (2)
Combination of the oxidative and reductive chemistry in photosynthesis gives eq. (3)
2H2O <-> 2H2 + O2 (3)
The change in energy can be estimated from the standard reduction potential E0’ (also known as the reduction-oxidation (redox) potential, or electrochemical potential) (see Box)
DE0’ = -1.23 V
This is equivalent to a change in standard free energy (representing the energy stored in the fuel (H2 or its equivalent in biomass) of
DG0’= 474 kJ mol-1
The subsequent reaction of this fuel with oxygen releases the stored solar energy in the reverse of equation (3), with DE0’ of 1.23 V and DG0’of -474 kJ mol-1.
Reduction potential
Reduction-oxidation reactions are the stuff of bioenergetics, and involve the transfer of electrons from one substance (donor) to another (acceptor) in accordance with their relative reduction potential. The reduction potential (also reduction-oxidation potential or redox potential) is the affinity of a substance for electrons. The value for each substance is compared to that of hydrogen, which is set arbitrarily to zero, at standard conditions of 25 C, 1 atmosphere, and 1 M concentration.
Substances that have positive redox potentials accept electrons from hydrogen becoming reduced, while substances that have negative redox potentials donate electrons to hydrogen, becoming oxidized.
The redox potential is also the same as the electrochemical potential and the Fermi level used in solid state physics [15].
The International Energy Agency, set up within the OECD (Organisation for Economic Co-operation and Development) during the 1974 oil crisis to address energy-related challenges in a collaborative manner, established its hydrogen programme (Hydrogen Implementing Agreement, HIA) in 1977. Included in the HIA is the photoelectrolytic production of hydrogen, which involved nine research groups from Japan, Sweden, Switzerland and the USA working together since 1999. A report published in 2004 said it has not achieved the ultimate goal of a stable sunlight-to-hydrogen conversion efficiency of 10 percent; but that goal was “in sight” [12].
The major roadblocks identified were as follows
I shall be describing some recent progress in overcoming these roadblocks in articles to follow, which will also explain artificial photosynthesis in more detail.
Article first published 29/06/09
Got something to say about this page? Comment
There are 2 comments on this article so far. Add your comment above.
sawitri Comment left 24th July 2009 15:03:07
very good. I will buy you book through kasetsart library.
François Comment left 27th April 2010 17:05:03
Bonjour,
je m'interresse au sujet dont traite votre article.. Je ne suis pas chercheur, je cherche à vivre en autonomie. Grace à vous j'ai compris ce que pourrait apporter la photosynthèse artificiel au stockage d'énèrgie solaire.
Peut aprés je suis tombé sur cet article:
Daizi Zheng, un designer chinois, a accouché de cette petite merveille écolo et futuriste : un téléphone que l’on remplit de soda (du Coca, sur la démonstration), afin qu’il y puise l’énergie nécessaire à son fonctionnement.
C’est, parait-il, en transformant en électricité les hydrates de carbone contenues dans les glucides du soda qu’il y parvient.
Qu'en dites vous?
Plus tard dans cet article il est dit que c'est grace a une enzyme que la transformation se fait.
Cordialement.
François