New flow batteries provide just the right energy storage for intermittent renewables such as solar and wind to guarantee constant supply throughout the grid Prof Peter Saunders
Electricity is a very convenient form of energy but it has the big disadvantage that it is hard to store. We cannot pile it into heaps like coal or keep it in tanks like oil or gas. Unless the amount is so small that it can be held in a capacitor (a charge-storage device), we have to turn it into another kind of energy and then back to electricity when we want it. There are a number of ways of doing this, such as turning it into gravitational potential energy by pumping water up hill and then letting it flow back down and drive a turbine when the electricity is needed, but by far the most common is to convert it into electrochemical energy, i.e. to store it in a battery. That works well for many applications, which is why batteries are so common, but not if there is a lot of energy to be stored. Think how large a battery you need to give a car a range of a hundred miles, compared with the much smaller petrol tank that will take it two or three times as far.
There has recently been a lot more interest in whether electricity can be stored efficiently because many countries are planning to generate more of their energy from wind and solar. Neither of these produces energy at a constant rate, still less at a rate that responds to changes in the demand for electricity. That’s not a fatal problem, of course, because there are other sources of energy such as biogas that can be used to fill the gaps. It is, however, still a serious issue, and forecasts of the cost of solar energy have built in the need for backup supplies. The Department of Energy and Climate Change, in predicting that 11.3GW of solar could be available in the UK as early as 2017, explains that that a key limiting factor is that the grid would need a substantial upgrading [1].
It’s not just a problem for green energy; even for conventional sources, it is not easy to adjust the supply to match the demand. While most conventional power plants can be run at any time of the day or night, they cannot be brought on stream rapidly, which means that the engineers who operate the system have to allow generous margins. They even have to estimate how many households will be roasting a turkey at the same time on Xmas or boiling a kettle for tea at the same break in a popular TV programme so that they can anticipate the extra load. The US Electric Power Research Institute estimates that a “smart grid” could lead to a 4% reduction in energy use by 2030, and efficient storage will be an important part of that [2, 3].
Over the past few years, batteries have improved considerably, and one of the most promising developments is the flow battery, which can store a large amount of electricity and also give the quick response that the power suppliers require. Some are already in use, and research is well under way into new and improved models. The most recent advance is the discovery that vanadium, the metal that is used in most of the current generation of flow batteries, can be replaced by a much cheaper organic molecule.
Most batteries are solid state. Some, like the common alkaline batteries, have a certain amount of energy stored in them when they are manufactured and have to be discarded (hopefully, recycled) when this has been used up. Others, such as the familiar lithium ion batteries, are rechargeable, though only a certain number of times.
The other battery most of us are familiar with is the lead-acid battery used in cars. This consists of two sets of plates in an electrolyte of sulphuric acid. In the charged state, the cathode (negative plate) is made of lead and the anode (positive plate) of lead oxide. When the battery is in use, the lead is converted to lead sulphate in a reaction that releases electrons. The lead oxide is also converted to lead sulphate but in a reaction that absorbs electrons. If the two electrodes are connected through a load, a current will flow. The battery is charged by supplying electricity to drive the reactions in the opposite direction. The energy is stored on the plates.
Lead-acid batteries are widely used, but they are too big and heavy to be used for storing large amounts of energy. An alternative is the redox flow battery, which operates on a somewhat different principle.
A redox flow battery has two different electrolytes separated by a membrane (Figure 1). Reactions similar to those in the lead-acid battery release and absorb electrons, but they take place within the electrolytes rather than in the electrodes. The membrane allows protons (hydrogen ions) to pass through but not larger molecules. Most of the flow batteries currently in operation use vanadium, a metal whose chief use is as an additive to steel. The electrolyte on the negative side contains two different ions of vanadium and the one on the positive side contains two different ions of vanadium oxide. Both are dissolved in acid.
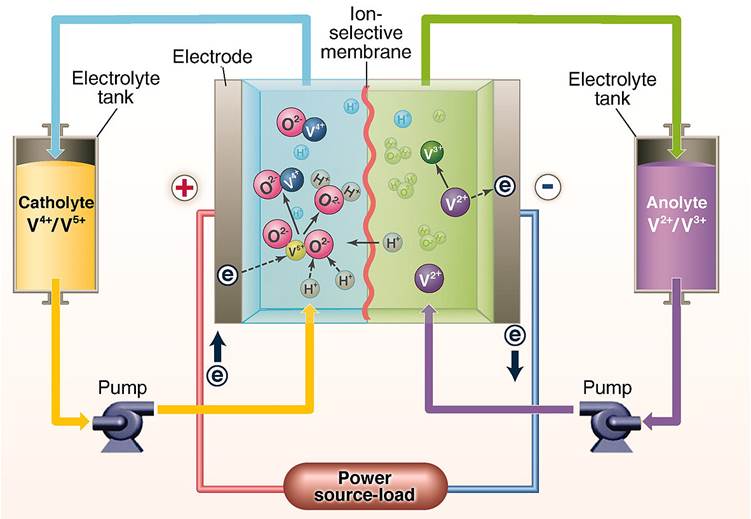
Figure 1 Flow battery with vanadium electrolytes
In charging, electrons are introduced to the negative side, converting V3+ ions to V2+, and removed from the positive side, converting VO4+ ions to VO25+. Hydrogen ions pass through the membrane from the positive side to the negative. When the battery is supplying energy (as is the case in Fig.1), these processes are all reversed.
The bulk of the electrolytes is held in storage tanks. While the battery is being charged, or while it is supplying electricity, the electrolytes are pumped through the cell where they interact electrically with each other and the electrodes. The battery can store a large amount of electrical energy because it holds it in the electrolytes rather than in the electrodes. The capacity is therefore limited by the size of the tanks, not that of the cell.
The lifetime of a vanadium flow battery is estimated to be about 20 years and the crucial component, the vanadium, does not degrade and is recoverable [4]. These features clearly contribute to the advantage these batteries have over other forms of storage.
Vanadium flow batteries are already in operation. In Japan, Sumitomo Electric has installed a large power generation and storage system at its Yokohama works. The system consists of an array of photovoltaic panels which can generate up to 200 kW, connected to a flow battery with a capacity of 1 MW × 5 hours. The battery allows the plant to make use of all the electricity it generates and to reduce the amount it takes from the grid when other demand is high [5].
The German firm Gildemeister has developed a redox flow battery that it advertises can be scaled up to multi-hour and multi-megawatt size. There are more than 50 in use in different countries, and Gildemeister has recently entered into an agreement with American Vanadium Corporation to market and sell the batteries in the US [4].
Vanadium is neither especially rare nor very expensive but the known resources are limited. The world’s chief supplier is China, and forecasters are predicting that if its use in flow batteries becomes widespread, the demand within China itself may limit the amount available for export and push up the price. Vanadium is also highly toxic.
Recently, a group at Harvard University have developed a redox flow battery that uses an organic molecule, a quinone, on one side and bromine and hydrobromic acid on the other (see Figure 2). The quinone, which is dissolved in water to form a quinone/hydroquinone couple, is 9,10-anthraquinone-2,7disulphonic acid (AQDS). According to the researchers it is almost identical to a quinone found in rhubarb, and can be synthesised from inexpensive chemicals [6, 7].
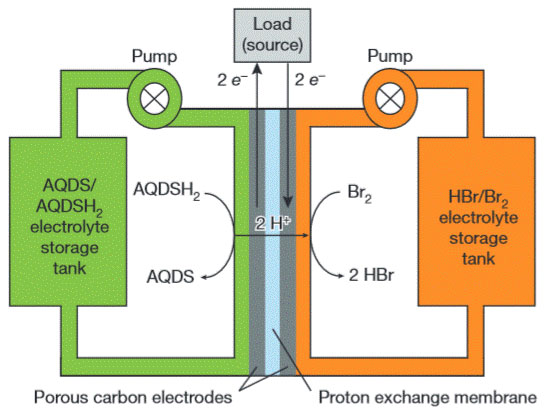
Figure 2 A flow battery based on organics
As in photovoltaics, battery technology based on modern science is rapidly improving and reducing our dependence on materials that are expensive or hard to source, with the result that the efficiency is increasing and the cost is falling. This is in complete contrast to nuclear energy, which has used the same science for fifty years and gets more expensive every year.
Flow batteries can store large amounts of electrical energy. They respond very quickly to changes in demand and can maintain their initial voltage for much longer than solid state batteries. This makes them suitable for ensuring that supply is closely matched to demand. They can increase the efficiency of conventional grids by allowing them to operate with a much smaller margin over the usual demand. They will be especially useful as solar and wind become major sources of energy, because having practicable and economical local storage will very much reduce the need for other sources of energy and for large grids to transmit it.
Article first published 12/03/14
Comments are now closed for this article
There are 6 comments on this article.
George Hewitt Comment left 13th March 2014 02:02:24
It is mentioned briefly "There are a number of ways of doing this, such as turning it into gravitational potential energy by pumping water up hill and then letting it flow back down and drive a turbine when the electricity is needed", and I am surprised that this method of storing energy is not more to the forefront of current discussions.
With the very high efficiency of modern turbines and pumps, this to me is such an obvious, flexible, scaleable and efficient (low running and maintenance costs) and very climate friendly.
All this quite apart from adding flexibile resource to the the increasingly stressed water/ agriculture resource in UK.
Heidi Schiele Comment left 14th March 2014 06:06:20
This should have taken place many years ago to save our planet. My hope is to apply this now without delay.
Alan Bangay Comment left 15th March 2014 02:02:04
What is the working life of a quinone molecule battery? Is it comparible to the vanodium baTTER? sINCE THE QUINONE CONTAINS WATER, IS IT SUITED fOR OPERATION IN LOW TEMPERATURES, SAY -30c OR EVEN LESS
jerilynn c. prior Comment left 15th March 2014 02:02:40
What are the size and weight of these flow batteries? I live in the wilderness and our only power (mainly for my laptop so I can work) is solar. Transport is a major issue. Hence my queston. Thanks, Jerilynn
Peter Saunders Comment left 15th March 2014 20:08:10
1. Quinone batteries are only just being developed so I don't think anyone knows what their working life is likely to be. Since the whole point is that quinones are cheap, they should be easy to renew. I doubt that quinone batteries will be suitable for -30C and below, but neither are vanadium batteries. Batteries are operated indoors, not out in the cold, so how they manage at such low temperatures probably doesn't matter.
2. The big advantage of flow batteries is that they can store large amounts of electricity. For small amounts, some kind of solid state battery would be more suitable. Don't you already run your laptop at night on the power it's stored during the day in its own lithium ion battery?
Todd Millions Comment left 19th March 2014 08:08:48
There are other alternatives that tend to have the advantage of incremental scale and disperable-Flywheels,in vacum chambers using magnetic bearings are being used in centralized substations settings-very rapid responce and 20 year life expectancies.Using extra power too run refrigerators to create ice that can be used for constant chilling loads-at least 2 orders of mag cheaper than storing electricity in batteries.But only a sheddable load,rather than power source.