Single or double layers of graphene can be precisely perforated to create molecular sieves with numerous applications from desalination to purification of water and gases and isolation of macromolecules Dr. Mae-Wan Ho
Defence contractor Lockheed Martin promises to revolutionize the process of desalination to get fresh water from seawater by filtering away the salt [1]. It will release a prototype of a membrane filter Perforene consisting of a single layer of graphene with selective molecular holes that will let only let water through under pressure, leaving the salt behind. The graphene membrane is much thinner than current filters, yet just as strong, if not more so; and requires a lot less energy to do the job. This could make a real difference to 780 million people in the world now living without access to fresh water.
So, what is the basis of the filtration process? It is simply graphene sheets with precisely controlled pore sizes.
The idea was first proposed in June 2012 by researchers at Massachusetts Institute of Technology (MIT) in the United States [2, 3] who carried out a molecular dynamics computer simulation.
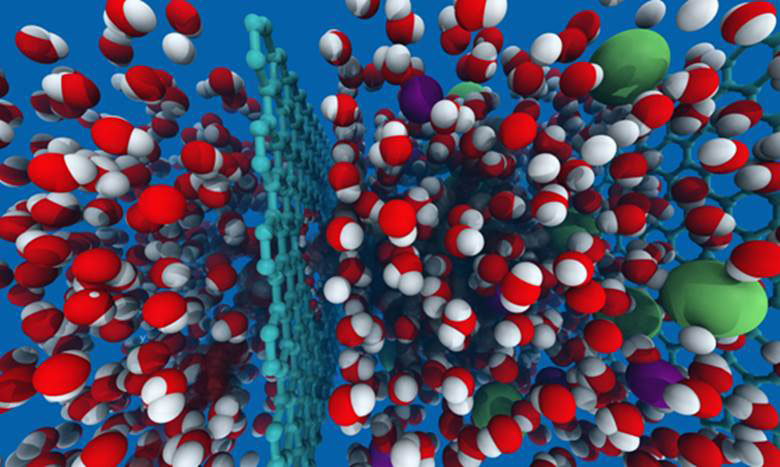
Figure 1 Artist’s impression of the desalination process with seawater containing sodium and chlorine ions (green and purple) on the right of the graphene film and only water molecules (red and white) on the left
One common current method of desalination is reverse osmosis, which uses membranes to filter the salt from water. These membranes are thick – about a thousand times the thickness of graphene - and require extremely high pressures. Still, it is the most energy efficient technique to date, with a record of 1.8 kWh/m3 achieved in a commercial plant in 2011 compared to about ~5 kWh/ m3 in the 1990s. Thermal desalination methods involving distillations are several times more energy-intensive [4]. Despite the widespread availability of seawater, desalination can only become a sustainable option if dramatically new techniques become available.
In their model, Jeffrey Grossman and graduate student David Cohen-Tanugi made precisely sized holes in the graphene sheet and also added chemical groups to the edges to interact with water molecules that either repel or attract them to see how the filtration process would work. Their results showed that water can flow across a graphene membrane at rates of 10 – 100 L/cm2/day at a pressure of 1 MPa (~10 atmospheres), and still reject all salt ions. This is 2 to 3 orders of magnitude higher than reverse osmosis. They also showed that for a given pore size, water permeability is significantly enhanced by hydroxyl groups alternating with hydrogen lining the pores compared with pores lined only by hydrogen. Pore diameter is critical, for pore diameter > 5.5 Å, the majority of salt ions approaching the pore entrance would be able to pass through the membrane. However, pressure is also important. For a perfectly rejecting membrane which excludes salt entirely, the operating pressure does not affect the exclusion. The results showed that salt rejection is close to 100 % for the smallest hydrogenated and hydroxylated pore, as well as for medium hydrogenated pore. For the remaining pores that shows some salt exclusion, the selectivity decreases both with pore size and applied pressure.
Will the actual graphene membrane work as predicted? Is it easy to reliably make graphene membranes that are large enough and strong enough?
Graphene is the strongest, and most stable material known. The single porous layer could be supported mechanically on a rigid porous layer to keep it from flexing or buckling, and there are techniques for making holes reliably and precisely in the membrane.
A research team led by Jeong Won Park at Telecommunications Research Institute, Kaist, in Korea, used a remarkably simple technique to reliably create a molecular sieve that separated a mixture of haemoglobin and immunoglobulin proteins in 10 minutes [4].
The technique relies on diblock copolymer lithography, which has been used for templating periodic arrays of holes on a molecular scale since the 1990s [5]. Block copolymers are made up of blocks of different polymerized monomers. The copolymer used is PS-b-PMMA, polystyrene-b-poly (methylmethacrylate), made by first polymerizing styrene, and then polymerizing MMA from the reactive end of the polystyrene chains. It is referred to as a diblock copolymer because it contains two different chemical blocks. The copolymer is first evenly spread on a supported layer of graphene by spin coating. The layer formed acts as a mask for reactive ion etching lithography, which takes advantage of the different chemical susceptibility of the blocks to remove one of them leaving the other standing before the etching reagents is applied. In this way they created a remarkable array of regularly space 15 nm holes in the graphene layer (Figure 2).
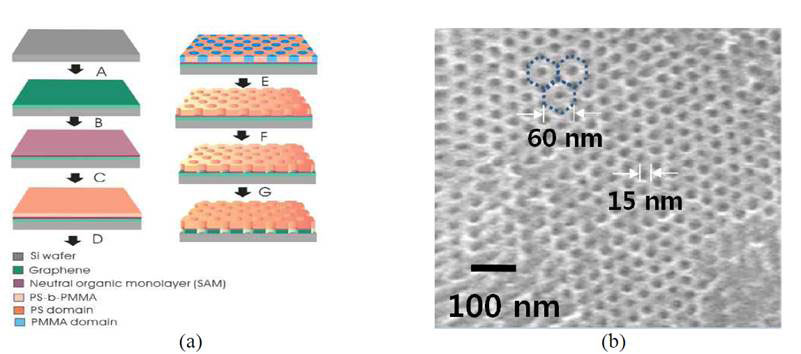
Figure 2 Di-block copolymer lithography for making molecular sieves (see text)
The three to four layer graphene molecular sieve was transferred to a SiN membrane with one micrometre-wide micropores that acted as support for the graphene membrane, and a separation test was carried out on the mixture of haemoglobin and IgG, each labelled with different fluorescent beads, at a concentration of 100 mg/mL each. The two proteins were separated in 10 minutes.
The authors concluded [6]: “Since the fabrication of the graphene nanosieves is so simple and easy to use and the separation of proteins works reasonably, the method can be applicable to the various medical devices, a fuel cell, and water purification.”
A research team at University of Colorado, Boulder, used ultraviolet-induced oxidative etching on a bilayer of graphene to create subnanometre holes that are highly selective for different gas molecules. They were not able to detect the holes directly under the atomic force microscope possibly because the density of the holes is quite low. However, they were able to test the selective permeability of different membranes using molecules of known sizes. Thus, one membrane allowed H2 and CO2 to leak through in several minutes, but not Ar (argon) N2, CH4 (methane) and SF6 (sulphur hexafluoride). As the kinetic diameter cutoff in the membrane is that of Ar (3.4 Å), the membrane has a nominal sieving kinetic diameter of 3.4 Å. In another membrane, all the gases leaked through in seconds except for SF6, which did not leak through at all; consequently, that membrane has a nominal sieving kinetic diameter of SF6, which is 4.9 Å.
Interestingly, UV-induced oxidative etching is just about the only thing that destroys graphene, making it lose its conductive and other electronic properties [7]. But this degraded graphene can still be used as molecular sieves.
Article first published 31/07/13
Got something to say about this page? Comment
There are 1 comments on this article so far. Add your comment above.
Todd Millions Comment left 25th August 2013 18:06:59
A uncle of mine decades ago saved and gave me a magizine for an article on airships(a 1975 Fortune).It also included a discription of a new way of punching very small holes in thin sheets for filter production on a molecular scale-using neutron beams from a research reactor.I then expected soon to be available mica(say)sheets,with nickel evaporated on one side,platinium on the other,with methanol sized pores punched thru, too be soon avaiavble for fuel cells.Didn't happen(till 1990's-manhattan scientific).Could such pores be set in graphene this way?Mayhap the Japanese should try-they have no shortage of neutron sources they are tripping over(literally).Not funny I know-weeping is useless at this point as well.Cheers