The structures are hundreds of microns or more in size and have the characteristics of soft matter; they bring quantum electrodynamics into the study of water, cell biology and medicine Dr. Mae-Wan Ho
Stable structured water is here. And it is best explained in terms of quantum electrodynamics field theory as pioneered by recently deceased Emilio del Giudice and his team. Emilio was the most brilliant, original, and best loved scientist of our time (see Emilio Del Giudice Prometheus of the New Science).
Solid structures tens of nanometres to millimetres in dimensions can now be seen under the transmission electron microscope (TEM) and the atomic force microscope (AFM), the most sophisticated in imaging techniques. All it takes is to allow drops of specially prepared water to dry at room temperature and pressure. Shui-Yin Lo, Head of Quantum Health Research Institute Pasadena, California, showed us numerous images at World Water Day Conference during our Colours of Water festival [1] (https://www.i-sis.org.uk/onlinestore/av.php); and further details were published in a paper with his son and lead author Alpha Lo [2].
The structures consist of clusters containing millions to billions of water molecules, and come in a profuse variety of shapes and sizes (Figure 1). They are flexible, and can be deformed by the tips of the atomic force microscope probe if scanned in the contact mode. Otherwise, the structures remain stable for weeks, even months, at ordinary room temperature and pressure. They have all the characteristics of ‘soft matter’ [3] - liquids, liquid crystals, colloids, polymers, gels, and foams - that form mesoscopic structures much larger than the molecules themselves, but small compared with the bulk material.
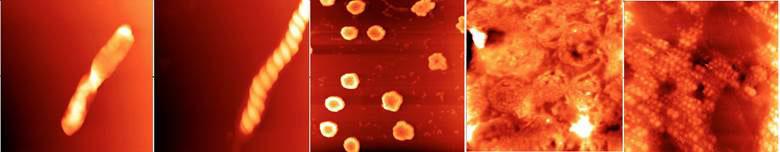
Figure 1 Different forms of supramolecular water clusters imaged with AFM; all fields are 5 microns square
Close-up, there appears to be a common fine structure to the clusters; they are all made up of small spheres tens of nanometres in diameter (Figure 1, right panel), lined up in strings that are further aggregated into rods (left panel) two of which wind around each other into a double-helix (second panel from left), or loops (middle panel) and wreaths (second panel from left). These and other observations suggest to Lo and colleagues that the sphere are dipoles (with separated positive and negative charges), enabling them to line up end to end to form an infinite variety of shapes and sizes. Significantly, no diffraction pattern characteristic of crystals was recorded, so neither the spheres making up the clusters nor the clusters are crystalline.
One interesting observation is that under the TEM, the rods appear dark (Figure 2), indicating that they diffract electrons strongly. The water was dried on a substrate of holey carbon film, as can be seen, some of the rods are curved (right panel) and in the middle panel, a rod was folded over the edge of a hole with half of it under the film, being flattened by the sharp edge, as consistent with its softness and flexibility.
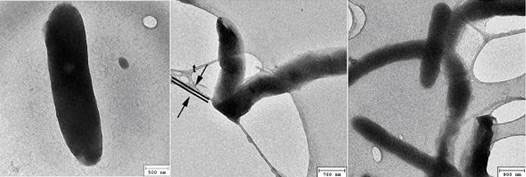
Figure 2 Dark rods under TEM
These amazing water clusters were discovered more than 15 years ago by Shui-Yin Lo, former high energy physicist. He “stumbled” into water research by accident after filing a patent with colleagues at the University of Melbourne in Australia on a new type of particle [4]. He was given leave of absence, and ended up a visiting professor at Caltech Pasadena to continue his work with the help of chemist David Gann.
Gann was then trying to develop a catalyst that would reduce smoke and pollutants in the oil-drilling industry, and came across one based on water, in fact, a ‘homeopathic’ dilution of a chemical catalyst much more effective than the catalyst itself. Intrigued, he enlisted Lo’s help, and there was no turning back.
Yin started to study all the available literature on water, and to carry out experiments and physical measurements. He and his colleagues began by publishing TEM images of the clusters in 1996 [5]. These “stable water clusters” were prepared simply by serially diluting a solution of 99.99% pure sodium chloride with vigorous shaking in ultrapure distilled de-ionised water in a low-dust room, then placing drops to dry on a clean glass slide or some other substrate [6]. The residue left behind is actually visible under an ordinary light microscope. As a control, drops of pure water were place on similar glass slides to dry under the same conditions, but no structures were seen.
The clusters are giant dipoles
To find out if the clusters are electrically charged, they were scanned backwards simultaneously on the AFM, that, instead of giving surface contours as in the forward scan, records voltages instead, acting as an electric force microscope (EFM). The results are shown in Figure 3 for a large cluster that looks a cloud. The voltage contours are almost a complete reversal of the surface contours.
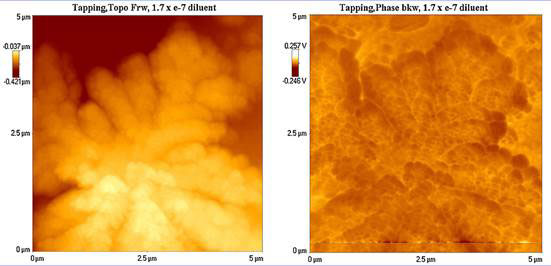
Figure 3 Surface contours (left) and electrical potential (right) of a large water cluster
Lo explained [4, 5] that in NaCl solution, when the density of (Na+ and Cl-) ions is high, the dominant interactions are among ions. As the concentration of NaCl decreases, interactions among ions are diminished, giving way to dipole interactions among water molecules themselves. The point at which dipole interactions dominate is found experimentally to be ~10-4M NaCl. At concentrations below this transition point, water molecules will attract one another to form clusters that have permanent dipole moment. A great variety of “snowflake-like shapes” were found. When clusters are not so dense, they spread out in straight lines, the horizontal interacting with the vertical at 102º (Figure 4). Remarkably, a distillate of the 10-7M NaCl also gives a cluster that is similar to that from drying a drop of the solution; indicating that the clusters remained intact (perhaps due to strong dipole interactions) upon heating, and furthermore, do not contain contaminating silicon or other ions.
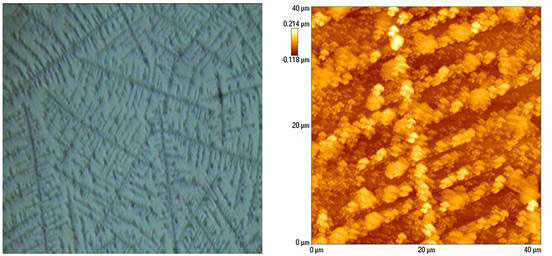
Figure 4 Large array seen under the ordinary light microscope (left) and atomic force microscope (right)
Spectroscopic measurements
The infrared spectrum of the clusters differs from that of pure water as does the Raman spectrum [6]. The water clusters could be detected in solution by photon autocorrelation using a helium-neon laser on a glass bottle containing the solution [7]. The interference between the light scattered by the clusters and the transmitted light enables the size of the clusters to be estimated. Three major sizes were centred at 15 nm, 300 nm and several microns. Interestingly, the clusters also formed with different initiating solutions such as isomaltose, cellulose, and sophorose, which could be seen on TEM.
To rule out the presence of contaminants being responsible for the clusters, some particles found in pure water were analysed with x-ray spectra compared with the water clusters. The particles in pure water registered a strong peak of Si, as well as Na, Zn Al, Cl, K, and Ca. In contrast, the water clusters were free of Si as well as the other ions.
The clusters could be observed under tapping mode on the AFM either dried or immersed in liquid water (indicating that the glass surface stabilizes the clusters). On AFM, the main sizes were 10nm, 100 nm and 1 000 nm.
Electric field in clustered water directs crystallization
Direct evidence of the electret (dipole) nature of the clusters was also provided by crystallization of monosodium phosphate NaH2PO4. The solid was dissolved in water containing the clusters and a small amount placed on a glass microscope slide and allowed to evaporate and crystallize. The slide was then examined with an optical microscope, compared with a control sample prepared in deionized water (Figure 5). The monosodium phosphate crystals from the solution containing clusters formed a pattern of straight radiating lines (Fig. 5a) while the crystals from deionized water failed to line up at all (Fig. 5b). Applying a small electric field of 20 V/in or 100V/in during the crystallization process did not have any effect on the deionized water sample (Fig. 5 d), while the crystals from the water with clusters aligned along the external field lines (Fig. 5 c).
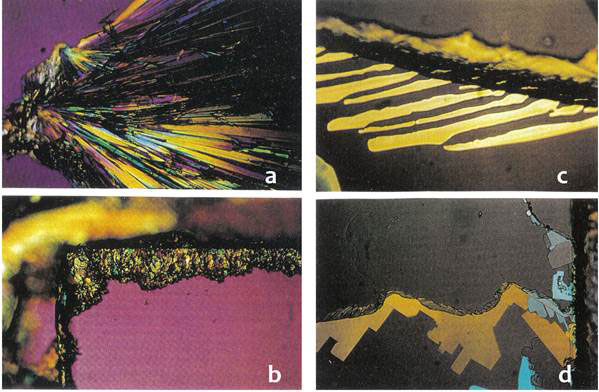
Figure 5 Crystallization of sodium monophosphate from solution with water clusters (a, c) compared with deionized water (b, d); a & c without external field, c & d in the presence of external electric field (rearranged from [7])
X-ray diffraction on powdered monosodium phosphate crystals formed from control deionized water and water containing clusters showed that while monohydrate was formed in deionized water from the original anhydrous sample, dihydrate crystals were formed in the water with clusters.
Deviation from linear relationship between pH and electric potential
An electric field of tens of mV could be measured between two stainless steel electrodes immersed in the solution with clustered water. In ordinary ionic solutions, no electric field is established between two identical electrodes. But in the water cluster solutions, the electric dipoles of the clusters can line up to establish an electric field without any electrochemical reaction occurring.
Using a pH meter, it is possible to measure the electric potential as well as the pH of a solution, and the two can be plotted against each other [7].
A wide range of pH from 1 to 12 was tested. The test solutions were prepared either with concentrated cluster solution or deionized water. The pH was adjusted with NaOH or HCl. The maximum deviation was found at neutral pH, where dipoles strongly dominate the system’s electric potential, and diminished at both ends when ionic contribution dominates the potential. The addition of KCl up to concentration of 0.01M to the cluster water solutions did not significantly change the shape of the pH vs electrode potential mV curve. Thus, the electric potential of dipoles was not affected by the presence of additional ionic species. In contrast, a linear relationship between electric potential and pH was found in deionised water that had no clustered water over the entire range of pH tested. The average difference in measured electric potential between ordinary water and cluster solutions was 92.4 mV for NaCl and 103.4 mV for KCl.
Structured clusters of water per se is not new; many different forms have been inferred in bulk water under ambient conditions beginning with the conventionally accepted H-bonded networks, the theory that water exists simultaneously in two states of different densities (for which there is now good evidence), the idea due to Martin Chaplin at South Bank University, London, UK, that the two states correspond to an expanded and collapsed form of a 280-molecule quasicrystalline icosahedral structure [8] (What is Liquid Water? SiS 58), and the remarkable interfacial water that forms a macroscopic exclusion zone (EZ) next to a hydrophilic surface, rediscovered by Gerald Pollack and his team at University of Seattle, Washington, in the United States (see extensive reviews in the two new books on water Living Rainbow H2O [9] by Mae-Wan Ho and [10] The Fourth Phase of Water by Gerald Pollack). (Both Chaplin and Pollack were speakers at the New Science of Water for Life Conference during our Colours of Water festival [11].) What’s new in the clusters isolated by Lo and colleagues are the stunning images of the actual structures and the high quality of the images obtained.
A team of researchers led by Antonella de Ninno at ENEA (Italian national agency for new technologies, energy and sustainable economic development) in Frascati, has also detected large water clusters in water that was repeatedly brought into contact with Nafion [12] in order to create water that have the same characteristics as EZ water, for example, in having an absorption peak at 270 nm in the UV region.
Fluorescence microscopy revealed large structures on which the polystyrene spheres appeared to be aggregated. Freeze-drying 20 ml of the Nafionated water gave 1 to 2 mg residue, and AFM confirms the presence of micron size structures superficially similar to those of Lo’s team.
The fact that a variety of initiators - both ionic and non-ionic (but polar) - appeared to create similar clusters suggests that water itself is the main determinant in the formation of both the clusters and of the EZ.
A full review of the literature surrounding structured water clusters, especially as described by Lo’s team, persuaded me that they are best explained in terms of special spherical dipoles created by coherent domains (CDs) that arise naturally from interaction between the ambient electromagnetic field and water [13]. The CDs were predicted by quantum field theorists led by Emilio DelGiudice, another speaker at our New Science of Water for Life Conference [14].
Standard quantum theory does not predict quantum coherence for liquid water, largely because it ignores both quantum fluctuations and the interaction between matter and electromagnetic field; these are only taken into account in quantum electrodynamics field theory. But conventional quantum electrodynamics field theory applies only to gases.
Del Giudice and colleagues extended conventional quantum electrodynamics theory to the condensed phase of liquids; they showed that interaction between the vacuum electromagnetic field and liquid water induces the formation of stable coherent domains (CDs) of about 100 nm in diameter at ordinary temperature and pressure, and these CDs may be responsible for all the special properties of water including life itself.
Each CD of water is a resonating cavity produced by the electromagnetic field that ends up trapping the field because water is much denser than air, so the frequency of the CD electromagnetic field becomes much smaller than the frequency of the free field with the same wavelength. Consequently, under ambient conditions, water is an approximately equal mixture of CDs surrounded by incoherent regions (see [9] for further details and original references).
Now, the small spheres of water (“balls”) that make up all the diverse structures of water clusters created by Lo’s team (Figure 1) have the dimensions of the CDs predicted, i.e., ~100 nm in diameter. This is in line with the finding that the precise nature of the initiator is unimportant, because it is largely a property of water itself, with the initiator molecules playing a catalytic role.
However, these spherical CDs are not dipoles in the ordinary sense of the word. Instead, quantum field theory predicts that the CD is oscillating between the ground state and an excited state of 12.06 eV, just below the first ionization potential of 12.56 eV, and therefore contains close to a million almost free electrons. At the same time, positively charged protons are extruded outside the domain, which is what happens also in the exclusion zones discovered by Pollack [10]. Del Giudice and colleagues hypothesize that EZ water is just a macroscopic coherent domain stabilized on the interface [15]. Consequently, these spherical CDs can mimic dipole interactions through negative charges on their periphery attracting positive charges just outside (see Figure 6) in a kind of three-dimensional perfectly symmetrical dipole, also referred to as an electret. There will be a dipole electric field measured in any direction. This metastable state will spontaneously break symmetry to favour one direction over all others, as when drops are placed onto a solid substrate, giving rise to a wide variety of aggregates or clusters. The proposed structure based on spherical dipoles from CDs with dense surface electrons should scatter electrons strongly and appear dark on TEM (Fig. 2).
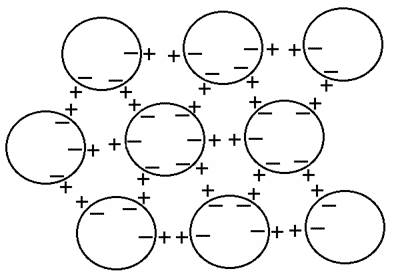
Figure 6 Spherical coherent domains forming a 3-dimensional dipole structure; note the 6-fold symmetry resulting from close-packing of sphere (see text for explanation)
Another prediction from quantum electrodynamical field theory as proposed by Del Giudice and colleagues [16-18] also favours the formation and stability of the structured water clusters. The coherent oscillations maintained by the electromagnetic field trapped within the CDs can occur not just between the coherent ground state and excited state of the water molecule, but also between two rotational levels, which produce correlations as large as several hundred microns, giving rise to a common dipole orientation, but a net zero polarization field (on account of its symmetry), unless and until the rotation symmetry is broken. The combination of the two coherent oscillations, therefore, produces phase-locked coherent interactions among the CDs, resulting in stable supramolecular clusters with the electret structure depicted in Figure 6.
Lo attaches special significance to the double-helix clusters (“double helix water”), which he believes – in the absence of real evidence – to be the precursor the double-helix in DNA and to be the basis of the acupuncture meridians of traditional Chinese medicine. He also believes double helix water is especially beneficial for health, because it [19] “works like a needle in acupuncture” (See an alternative, though not mutually exclusive hypothesis on acupuncture meridians based on superconducting water aligned with collagen fibres that I have proposed [20]). Impressive changes to the thermography images of the meridians can be observed before and after drinking double helix water, and there are preliminary results suggesting that double helix water may be beneficial for autism, diabetes, thyroid, brain, and digestive systems [19, 21, 22]. Lo and his team have opened up an exciting avenue for future research that could indeed revolutionize biology and medicine (see also [9] and [10]), together with the work of other scientists featured at our Colours of Water art/science/music festival (https://www.i-sis.org.uk/coloursofwater/).
A more technical fully referenced version of this report has been accepted for publication and is in press in the Water Journal (http://www.waterjournal.org/
Article first published 12/02/14
Got something to say about this page? Comment
There are 2 comments on this article so far. Add your comment above.
braith k osborn Comment left 13th February 2014 19:07:44
The next step must be the interaction between double helix water and ribonucleotides.
Can we produce helical RNA
Todd Millions Comment left 16th February 2014 19:07:32
Please explain-"water drops allowed to -dry -at STP"(paraphrase).Given the metastable state,have other structural topologies being considered,as in Fullere's vector equalibriam Jitterbug model?*
Very interesting report,Thanx.
*Synergetics