An electric property universal to all cells turns out to determine their vital states, from cell division and pattern formation to differentiation, regeneration, and cancer; lending support to the electrodynamics basis of life Dr. Mae-Wan Ho
The membrane potential refers to the electrical potential difference across the cell membrane: the inside with respect to the outside. When the cell is ‘at rest’ in a steady state, its ‘resting’ membrane potential averages about -50 mV, with a range of -10 to -100 mV.
Although a great deal of work had been done on the nerve cell, pioneered by British physiologists and biophysicists Alan Hodgkin (1914-1998) and Andrew Huxley in the 1950s [1], focussing on the action potential - an event in which the membrane potential rapidly rises and falls - the membrane potential of other cells has been relatively neglected.
Within the past decade, however, the membrane potential of a wide range of cells has been coming under scrutiny, thanks to voltage-sensitive dyes that fluoresce or change colour according to electrical potential. Researchers can now follow electric potential changes both of large populations of cells and of localized patches of membrane or organelles in a single cell. These potential changes appear to determine vital states - from cell division and pattern formation to differentiation, regeneration, and cancer [2-6] - lending considerable support to the idea that cells and organisms intercommunicate and coordinate their activities coherently by means of electric and electromagnetic fields (see [7] The Rainbow and the Worm, The Physics of Organisms, ISIS publication). In this article, I shall concentrate on development and regeneration.
Research on bioelectric activities of cells in general actually goes back at least as far as the 1930s (see [7]), but never quite reached mainstream status. In the 1970s, Lionel Jaffe (1918-2011) and Richard Nuccitelli, then at Purdue University Lafayette, Indiana, in the United States, pioneered the vibrating probe technique for measuring electrical currents noninvasively near individual living cells [8]. This led to the discovery that all developing embryos drive ionic currents through themselves. These ionic currents are thought to be responsible for electric fields generated inside the embryos, which had been discovered earlier using microelectrodes. Electric fields ~20 mV/mm have been measured in chick and frog embryos using microelectrodes [2]; and perturbing these electric fields results in developmental abnormalities. Mammalian skin wounds, similarly, generate large fields of up to 150 mV/mm right next to the wound, and reducing or augmenting these fields could delay or accelerate healing.
It has been known since the 1950s that the membrane potential varies throughout the cell cycle [3]. Cell types with very high resting potentials such as muscle cells and neurons show little if any tendency to divide, while a decrease in membrane potential follows malignant transformation, in which cells multiply out of control. In the 1970s, Clarence D. Cone Jr. induced DNA synthesis and mitosis in fully differentiated neurons from the central nervous system using a variety of agents that depolarized the cell membrane (made it less negative) [9]
Microelectrode and related patch clamp techniques are difficult and time consuming, and can only measure the membrane potential of an extremely local area. Voltage sensitive dyes are much easier to use and can give information on spatial and temporal variations of membrane potentials over large areas or populations of cells (though it is difficult to determine the actual electric potentials without first standardising colour changes against measurements made with microelectrodes). As a result, new vistas have opened up for bioelectricity research; especially for scientists at Tufts University, Medford, Massachusetts in the United States, studying stem cell growth and differentiation, cancer, and embryonic development.
Stem cells are the key to regenerative medicine (see [10] The Promise of Induced Pluripotent Stem Cells, SiS 51). However, one major difficulty is in controlling their growth and differentiation; and it has become clear that the misregulation of stem cells can lead to cancer [11]. There is an intimate connection between correct differentiation of stem cells during development and in tissue replacement. Michael Levin and his team at Tufts University perturbed stem cell differentiation in the developing frog in order to throw light on what makes stem cells multiply out of control.
In vertebrates, the ‘neural crest’ is a population of embryonic stem cells that form many structures including smooth muscle cells, peripheral neurons and glia, cartilage and bone in the head and face, as well as endocrine and pigment cells. Neural crest misregulation gives rise to an important class of birth defects in humans. Neural crest cells not only reveal dynamics of cell migration, but are important for understanding melanoma, a malignant cancer of melanin-producing cells.
Armed with the knowledge that depolarization of the membrane potential leads to cell proliferation, and that depolarization of the neural crest cells can be achieved by targeting the glycine-receptor chloride channels (GlyCl), which has been previously achieved in vitro, the team carried out the experiment in vivo on developing frog embryos [12].
Ivermectin is a drug commonly used as an anti-parasite agent and known to specifically open GlyCl. Keeping chloride channels open leaks Cl- into the medium, thereby depolarizing the cell (making it less negative).
Untreated Xenopus (African clawed frog) embryos develop a characteristic pattern of pigmentation after a fraction of the neural crest cells in the early embryo become melanocytes during the later neurula and somite stages, and begin to produce melanin during the still later tail bud stages. The pigmented cells are largely confined to the mid region in the head and trunk of the young tadpole (Figure 1, top). In contrast, embryos treated with ivermectin showed extensive hyperpigmentation, the melanocytes migrating to regions normally devoid of pigment cells (see Fig. 1, middle). This happened to 98 percent of the embryos treated. The treated embryos were paralyzed as tadpoles, as expected from the depolarizing effect. Development was otherwise normal, and no other organ or tissue derived from neural crest cells was affected. Thus, opening the glycine chloride channels in embryos specifically resulted in hyperpigmentation.
Ivermectin hyperpigmentation also involved inappropriate cell migration, as blocking melanocyte movement with metalloprotease inhibitor NSC-84093 resulted in tadpoles hyperpigmented only in the mid regions (Fig. 1 bottom). Otherwise, the migration of the abnormal melanocytes was extensive; they colonized not only the lumen of the neural tube, but also penetrated the dense neural tissues, and sent long projections from the edges of the somite (blocks destined to become trunk muscles) into the neural tube. They are reminiscent of metastasis (spreading of cancer cells).
To prove that the ivermectin effect was due to opening the GlyCl, the embryos were exposed to the normal binding partner of GlyCl, glycine, which binds to the protein at a different location from ivermectin. Treatment with glycine induced the same hyperpigmentation as ivermectin.
To identify which cells expressed the ivermectin target, in situ hybridization with an antisense probe for the mRNA of GlyCl a-subunit (to block its expression) confirmed that the GlyCl was expressed exclusively in the neural-crest area in the early embryo, and later in a sparse peppered pattern throughout the embryo, but not expressed in the melanocytes themselves.
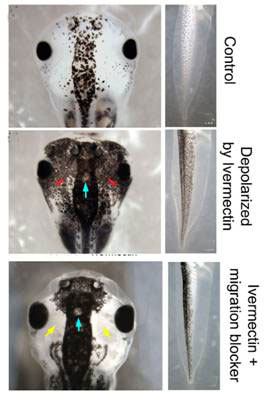
Figure 1 Depolarization of membrane potential and hyperpigmentation
To show that the effect of ivermectin was due to depolarization of the membrane potential rather than some other alteration of GlyCl function, chloride concentration in the external medium was increased from 10 mM to 30, 60 and 90 mM. As the internal chloride concentration can be as high as 60 mM, increasing the chloride in the external medium will reverse the depolarization. Indeed, the hyperpigmentation effects were partially suppressed at 60 mM and completely inhibited at 90 mM chloride in the external medium.
Finally, membrane depolarization resulting in hyperpigmentation could be achieved by blocking other proteins, for example, by injecting the non-functional mutant of the ductin subunit of V-ATPase pump, which hyperpolarizes cells.
Thus, it is a change in membrane potential as such that causes the effect, a result that’s repeated for many other functions investigated by the researchers.
The most exciting serendipitous new discovery of the Tufts University researchers is the ‘face’ of the frog roughed out in membrane potential differences very early in development when the embryo is still a shapeless ball of cells with very few anatomical features [13].
A team led by Dany Adams used a combination of voltage and pH sensitive dyes to follow the development of Xenopus embryos under a microscope fitted with a time-lapse camera. It recorded “never-before-seen” dynamic patterns of membrane potentials on the outermost cell layer evolving in roughly three sequences (see Figure 2). These are clear signs of electrodynamical processes determining structures that appear much later on.
The first (Fig. 2A, Course I) is a wave of hyperpolarization (more negative membrane potential) sweeping across the entire embryo in about 15 minutes at stage 13, when the embryo is a gastrula, a double layer ball of cells.
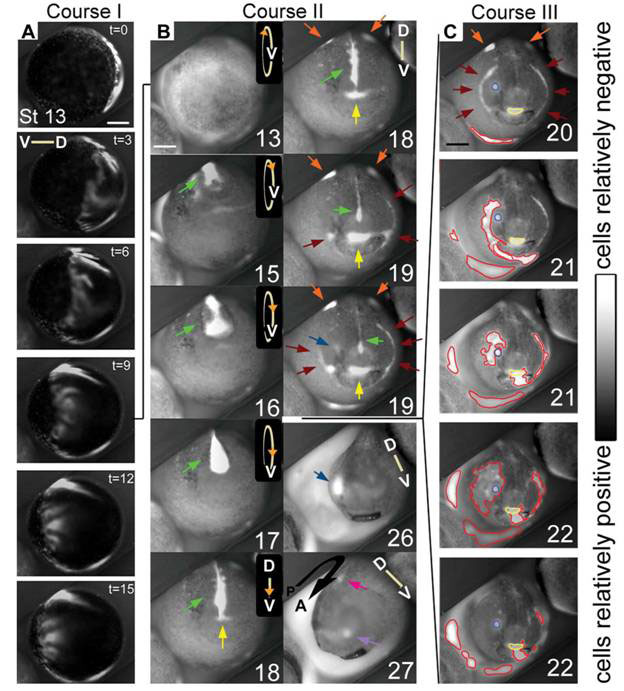
Figure 2 Dynamic patterns of membrane potentials in the developing Xenopus embryo
(D, dorsal, V, ventral)
The second (Fig 2B, Course II) following the first, coincides with the closure of the neural tube (the future spinal cord and central nervous system) in which a bright line of hyperpolarization coming from the median ectoderm (green arrows) gets occluded as the folds close over it, while somewhat dimmer patches appear at the lateral ectoderm (orange arrows). As the neural tube closure ends, distinct bright spots and lines of hyperpolarization appear in anterior areas that subsequently invaginate (sink inside the embryo). These hyperpolarized regions mark out the future mouth area (yellow arrows), the cement glands (brown arrows) the nose area (lavender arrows) and the first pharyngeal fold (brown arrows), the eye field (blue arrows) and the future ear (magenta arrow). At some point, the patterns do take on an eerie semblance of a face (stages 18 and 19), though except for the mouth and the pharyngeal folds, the other facial features are not mapped out in their normal positions of what one would recognize as the ‘face’.
The third sequence of bioelectric activities (Fig. 2C) takes place after the closure of the neural tube and is coincident with the elongation of the embryo (along the anterior posterior axis). It consists of an embryo-wide series of localized hyperpolarizations, less orderly than the first two, forming and spreading in small areas that sometimes overlapped with the regions established during the second sequence.
In the same report, the researchers showed that the development of head features depends on the proton transporter protein H+-V-ATPase, which exports protons (H+) from the cell using ATP. Many V-ATPases are found on intracellular vesicles but the ones involved in head development are found embedded in the cell membrane. By pumping H+ out, it hyperpolarizes the cytoplasm, making it more negative. Disrupting this enzyme by chemicals or injecting mRNA that makes faulty H+-V-ATPase led to many head and face abnormalities.
The H+-V-ATPase was identified as the result of a hierarchical screening procedure with drugs that inhibit progressively more specific ion transporters that block the requisite change in membrane potential for the function under investigation. The Tufts group refers to this specialty as “chemical genetics” [3, 4], which enables them to make good use of genomics information.
However, exposure to other agents that altered membrane potential by preventing hyperpolarization or preventing the cytoplasm becoming less acid (from loss of H+) gave the same abnormalities; while another proton pump that normally sits in the cell membrane can compensate for the loss of H+-V-ATPase function. These findings again indicate that it is membrane potential as such that appears to determine head and face formation at a critical time, upstream of many of the genes identified as crucial for head and face formation.
Young Xenopus tadpoles readily regenerate their tails when cut off. That too, depends on the proton pumping activity of the H+-V-ATPase [6], but only through the change in membrane voltage, “an early mechanism necessary and sufficient to induce tail regeneration [14].
Xenopus is not alone in depending on membrane potential for embryonic development and regeneration
The planarian flatworm is a favourite model organism for studying regeneration, even more so than Xenopus. When it is cut in half, the part that has the head regenerates the tail, and the tail half regenerates the head. And even when it is chopped up into more pieces so that some of the pieces have neither head nor tail, the fragments can still remember which side was nearer to the head and which nearer the tail, and regenerate both head and tail in the correct orientation.
The adult planarian Dugesia japonica has adult stem cells (neoblasts) for replacing differentiated tissues lost during normal turnover of cells. When the adult is amputated, these neoblasts proliferate and migrate to restore the missing parts by forming a regenerative mass, the blastema, which eventually differentiates into the missing structures. Previous research indicates that crucial determinations at the site of injury take place during the first day of regeneration and involve signals from both local and distant tissue. Among proteins already implicated in the correct specifications of structures during regeneration are gap junction channel proteins located in the cell membrane and involved in direct cell-cell communication.
In addition, literature dating back to the 1950s and 1960s suggests that the nervous system is an essential component of regeneration in both vertebrates and invertebrates, but the mechanism remains poorly understood.
Michael Levin’s team began by screening for gap junction (GJ) blockers [15]. In planarians, treatment with known GJ blockers heptanol or octanol, but not hexanol (which does not block GJ) led to consistent alteration of anterior/posterior (AP) polarity during regeneration. The researchers optimized the dose of octanol so it induced consistent AP polarity alterations without blocking all gap junctions simultaneously, which would otherwise poison the organism and the neoblasts.
A series of transverse cuts were made to give fragments cut at both ends along the AP axis; the fragments were then treated with octanol or left untreated. Both the untreated controls and the octanol-treated fragments developed normal anterior blastemas that gave rise to heads. However, fragments treated with octanol often formed anterior blastemas at the posterior-facing ends that developed into heads, resulting in animals with heads at both ends, an abnormality never found in untreated animals. The tendency to developing heads at both ends (bipolar head) gradually increased as the plane of amputation moved towards the posterior end, starting from the head, reaching maximum (~100 percent) just behind the pharynx (see Figure 3).
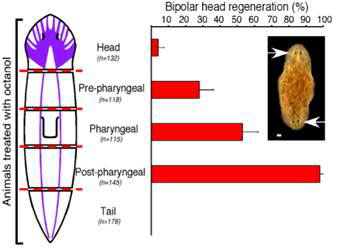
Figure 3 Double headed regeneration after octanol treatment
Although previous work showed that planarian GJ proteins, innexins, are important for stem cell function and blastema formation, it remains unclear which, or how many of the dozen innexins are actually involved in AP patterning during regeneration. Michael Levin’s team used RNA interference, injecting short stretches of double stranded RNA to target genes in a sequence-specific manner, to silence the genes in the adult planarians before they were amputated. They targeted up to four innexins simultaneously by injecting more than 600 animals with the iRNA combinations, and narrowed down to three, which when blocked simultaneously resulted in abnormalities similar to those caused by octanol: Dj-Inx-5, Dj-Inx -13, and Dj-Inx-12. These genes are expressed in the central nervous system as well as in sub-epithelial cell populations throughout the animal, and are up-regulated within the regenerating blastema. Injecting the iRNAs of these genes into intact animals caused behavioural changes as well as inversions of the AP polarity, with extra pharynxes growing at inappropriate places.
The planarian brain can prevent regeneration of secondary heads within the same animal. Thus, the head fragment produces the fewest double headed animals. This inhibition can act at long range. As any fragment containing the head, however far away the head is from the cut end, will rarely give rise to double headed animals when treated with octanol.
The researchers suspected that the ventral nerve cord was responsible for inhibiting head formation in the presence of octanol. To test the idea, they made cuts that did or did not disrupt the ventral nerve cord (VNC) along the AP axis. All treated and untreated animals formed blastemas, but those with interrupted VNC treated with octanol developed multiple AP axes, with heads, pharynxes and protrusions in the wrong places (see Figure 4).
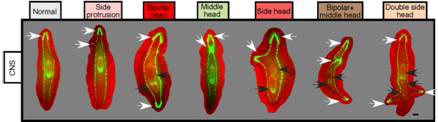
Figure 4 Abnormal AP axis with octanol treatment and VNC disruption
Do the central nervous system and the GJ signals also affect healing or regeneration of wounds on the sides of the body? To answer this question, the researchers made lateral cuts in post-pharyngeal fragments treated with octanol that had the VNC disrupted, or not. All cuts resulted in blastema formation; and to their surprise, all exhibited behavioural changes as if they are being pulled in different directions simultaneously, and photoreceptor pigments (normally present in the eyes) appeared in two, three or four different blastemas within the same fragment, suggestive of rudimentary heads. And three or four heads did eventually form in their fragments (see Figure 5). Thus, the CNS and GJ provide signals to both posterior and lateral wounds to inhibit head formation.
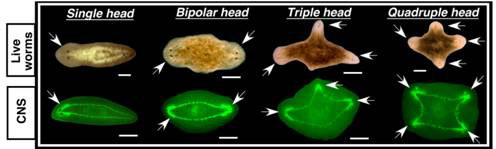
Figure 5 Multiple heads from lateral wounds treated with octanol
To find out when the central nervous system and the GJ proteins are needed for correct specification of the AP polarity, the researchers carried out treatment at different times after amputation. Fragments without heads treated with octanol (GJ inhibitor) gave the most significant effects within the first 3-6 hours after amputation, dropping to less than 60 percent for treatment beginning more than 12 hours after amputation. For VNC disruption, a dramatic, 4-fold decrease in the incidence of AP defects occurred after 3 hours post-amputation. These findings suggest that both GJ proteins and the central nervous system act early during regeneration to correctly specify the missing structures.
Amazingly, animals with two, three or four heads regardless, not only survive, but can regenerate the same acquired pattern on further amputations in the absence of octanol. It appears that a single treatment is enough to reset the AP axis, and the memory of that can now be perpetuated indefinitely. This is a genuine inheritance of acquired characters, and does not involve any change in the genetic material; the amount of octanol used in the original experiment was not mutagenic. It is reminiscent of a ’morphogenetic field effect (see later).
In a follow-up study, the researchers uncovered, once again, that membrane depolarization, mediated by H+, K+-ATPase, is essential for anterior gene expression and brain induction [16] (Figure 6). As in Xenopus, independent manipulation of the membrane potential with ivermectin confirms that depolarization drives head formation, even at posterior-facing wounds.
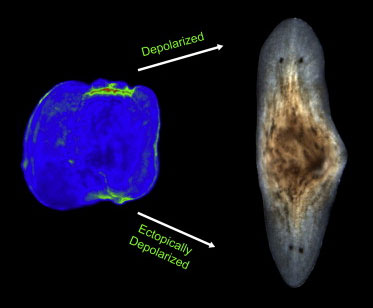
Figure 6 Depolarization means heads
Decades of painstaking molecular genetic analysis followed by genomics have succeeded in mapping out detailed pathways of gene induction and repression in the formation of different body structures during development. Despite that, there is little progress in our understanding of how patterns and forms are generated, starting with an almost entirely featureless egg or cell mass. The patterns of specific gene expressions follow in the wake of pattern determination processes, which invariably include a change in membrane potential.
However, identifying membrane potential changes is only a start, for one must also ask how a localized membrane potential change arises in the first place. And following that, how more complicated patterns, such as repeated body segments, limbs, shoots and roots are determined. We shall examine all these questions next ([17-19] Genes don’t Generate Body Patterns, Liquid Crystalline Morphogenetic Field, and Electronic Induction Animates the Cell, SiS 52), in order to flesh out the electrodynamical nature of life.
Article first published 21/09/11
Got something to say about this page? Comment
There are 4 comments on this article so far. Add your comment above.
Brigitte Hansmann Comment left 22nd September 2011 06:06:09
Dear Mae Wan, thank you very much for your work! It is most valuable for my work as a clinician in dfa somatic pattern recognition. I am looking forward to the three articles you announce.
Best wishes,
Brigitte
Kaviraj Comment left 22nd September 2011 06:06:00
It is in .this context interesting to compare the work of G.W. Crile in 1926, when his seminal book on this very subject was published. "A Bi-polar Theory of Living Processes" was its title. Well reasoned out and with clear illustrations in colour and black and white, it make a strong case for the electromagnetic basis of vital-force=defence-system and its implications for all sciences.
In homoeopathy we already use electromagnetic means of cure. Boyd of Glasgow (1946) and Abrams of the US (1930's) built machines that could read the level and provide the matching remedy.
Glad to see it making a comeback in more mainstream thinking.
Todd Millions Comment left 22nd September 2011 17:05:15
The field map of electrical potential(normally),Becker(Robert O-see;Body Electric)described was(if I'm remembering correctly)-Positive at centre,and negitive charge at the extremities.This was I seem to remember re enforced in vertabrates by the diode peizo electric currents generated by (living) bone.
Would this orientate this feild?
Rory Short Comment left 22nd September 2011 18:06:37
I have never studied the life sciences but have always been fascinated by life approaching it at a more macro level I suppose. Despite my lack of knowledge at this more micro level I found this article very exciting. It is great that knowledge gleaned at this micro level is beginning to provide a hard science foundation for previous understanding that was in a sense arrived at more intuitively.