Solar Power
Solar power could become the next big thing in homes, personal accessories, the battlefield and other military applications
New affordable, durable and portable solar devices provide local energy generation for maximum efficiency and minimum greenhouse gas emissions. Dr. Mae-Wan Ho
Organic solar cells emerged in the late 1970s, based on conjugated polymers – polymers with alternating double and single carbon-carbon bonds – when it was discovered that doping these materials - slightly contaminating with appropriate chemical elements - increased conductivity several orders of magnitude [1]. Since then electronic conducting materials based on conjugated polymers have found many applications including light emission diodes (LEDs) and solar cells.
Nowadays, organic semi-conductors include not only polymers (molecular mass more than 10 000 atomic mass units), but also small molecules (molecular mass less than a few thousand units), and dendrimers, with molecular masses in between the polymers and small molecules. The distinctions between the different kinds of molecular semiconductors are important in determining the processes required in making films and devices, but the way they work is identical.
Organic solar cells work differently from conventional inorganic semiconductor solar cells. Light absorbed by an inorganic semiconductor produces free charge carriers – electrons and holes – that are transported separately through the semiconductor material. In an organic solar cell, however, light absorption produces excitons, electron-hole pairs that are bound together and hence not free to move separately. To generate free charge carriers, the excitons must be dissociated. This can happen in the presence of high electric fields, at a defect site in the material, or usually, at the interface between two materials that have a sufficient mismatch in their energy levels.
Thus, an organic solar cell can be made with the following layered structure: positive electrode/electron donor/electron acceptor/negative electrode. An exciton created in either the electron donor or electron acceptor layer can diffuse to the interface between the two, leading to electron transfer from the donor material to the acceptor, or hole transfer from the acceptor to the donor. The negatively charged electron and the positively charged hole is then transported to the appropriate electrode.
Organic materials are diverse and versatile, offering endless possibilities for improving a wide range of properties such charge generation, separation, molecular mass, ‘wettability’ (between organic molecules and inorganic material), bandgap (determining the ability to harvest light efficiently in different parts of the solar spectrum, especially the infrared), molecular energy levels, rigidity, and molecule-to-molecule interactions. Different organic molecules can be combined with one another, or with inorganic materials in many unique, favourable formulations.
One major advantage of organic solar panels is the low cost involved in manufacture. Organic molecules are cheap to make, they can have very high light absorbing capacity so that films as thin as several hundred nanometres would be sufficient for the purpose. Organic materials are compatible with plastic and other flexible substrates; and devices can therefore be fabricated with low-cost, high throughput printing techniques that consume less energy and require less capital investment than silicon-based devices and other thin-film technologies. One estimate put the reduction in cost by a factor of 10 or 20 [3]. Consequently, organic solar cells do not need to have conversion efficiencies as high as thin-film inorganic solar cells to become competitive in the market.
Organic materials can be printed on in any pattern or colour, and integrated into existing building structures, or even clothing or other accessories. In a couple of years, we are told, it will be possible to recharge one’s mobile phone from one’s jumper, or power up one’s laptop by plugging into the beach tent.
Seriously these affordable new generations of solar devices will be a boon for the energy needs of poor countries that do not have power grids or other infrastructure support. Generating electricity for use on site also avoids the huge losses incurred in generating electricity in power stations and distributing through the grid, estimated to be as high as 69 percent [3]. This is why local ‘microgeneration’ of electricity is also gaining favour in developed countries as a means of improving on efficiency and minimising greenhouse gas emissions.
Most organic solar cells are currently running at conversion efficiencies less than 5 percent. These include flexible thin-film modules made of light-harvesting organic plastic polymers.
Kornaka Technologies, a company based in Lowell, Massachusetts, USA, announced the acquisition of Siemens’s organic photovoltaic research activities in September 2004 in order to develop and commercialise new plastic power cells [4] for “any electronic device or structure to carry its own on-board source of renewable energy.” Siemens has its headquarters in Berlin and Munich, and is one of the world’s largest electrical engineering and electronics company. Konarka cofounder and CEO Howard Burke said that the company was testing various product applications for consumer electronics and military devices at its pilot manufacturing facility in Lowell. However, he would not say whether the tests have reached the company’s stated goal of 10 percent efficiency [5].
A year later, Konarka announced a joint research programme with Evident Technologies, a company based in New York USA, to develop “ultra high performance plastic solar cells” [6] that combine its novel polymers with Evident’s quantum dot nanotechnology. The quantum dot power plastic could be used for “demanding energy, communications and military applications, such as battlefied or off-grid power generation.”
The Pentagon is hoping to use Konarka’s solar cells to create a tent that can generate electricity from the sun, and tools that soldiers can carry in the field to recharge the batteries in their cell phones, night vision scopes, and global positioning systems.
A major problem with the plastic solar cells and organic solar cells in general is stability and longevity. Apart from chemical decomposition of the organic molecules, organic solar devices can degrade from distortion, loss of adhesion of the layers, or the layers diffusing into each other. So, careful design of the device and engineering more stable molecules are needed to substantially improve the lifetimes of the device. Rapid progress has been made on these fronts, especially with an organic-inorganic hybrid solar cell.
Dye sensitised solar cells (DSSCs) are among the third generation devices nearest to the market, or already in the market. These are not purely organic solar cells, but are made of a hybrid of organic and inorganic semi-conducting materials.
The basic scheme of a DSSC is shown in Fig. 1 [7]. A layer of light-sensitive dye is attached to the surfaces of the 15-20 nm nanoparticles of TiO2 in a 5-20 microns thick film. One main effect of the nano-structured film is to greatly amplify the light-sensitive surface. The actual surface area in a 10 micron thick film is 1 000 times greater than that projected; because of the small size of the particles, these films are highly transparent. To help enhance light harvesting in the red and near infra-red range, quantum dots of 100 nm to 400 nm are incorporated into the TiO2 film (see “Quantum dots and ultra-efficient solar cells”, this series). The TiO2 film is deposited by screen-printing from a colloidal suspension and sintered (heated to a high temperature to fix it).
The dye is a member of a class of red ruthenium complexes code named N3, or N719. The dye-sensitized photocell shows a broad photon-to-current efficiency – the number of electrons generated per photon striking the cell - of more than 70 percent between 350 to 660nm. The excited electrons are injected very rapidly (10-15-10-12 s with 100 percent quantum efficiency into the conduction band of the TiO2. (The quantum efficiency is the fraction absorbed by the dye that is converted into conducting electrons.) Dye regeneration occurs in 10-12 s, an order of magnitude slower than electron injection, while charge recombination takes place even slower, on a millisecond timescale, which means they do not interfere with efficient charge separation [8].
The dye has been optimized for its light absorption characteristics as well as stability. It is stable enough to sustain about 108 turnover cycles, corresponding to about 20 years of exposure to natural light, which is longer lasting than amorphous silicon. The most recent record in power conversion efficiency set by a cell of this type is 11 percent, in the laboratory of the inventor Dr. Michael Gratzel in Lausanne Switzerland [7]. This is considerable progress from an efficiency of 1 to 2 percent reported in 1988.
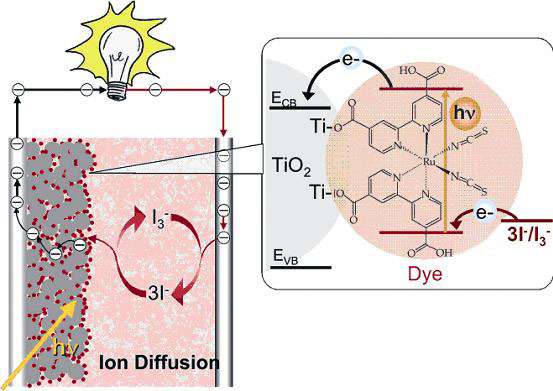
Figure 1. A dye-sensitized solar cell. The gray dots represent nanoparticles covered with a single layer of dye (small red dots). Electrons are represented by circled minus signs, an incident photon absorbed by hv. ECB and E VB are the energy levels of the conduction band and valence band respectively, which defines the band gap. Redrawn from [7]
Some recent milestones in improving stability and efficiency include turning the liquid electrolyte into a gel, thereby preventing leakage of the electrolyte from the cell. The cell sustained heating for 1 000h at 80C, maintaining 94 percent of its initial performance. The device also remained stable under light soaking at 55C for 1 000h in a solar simulator equipped with an ultraviolet filter. This cell had a conversion efficiency of more than 6 percent [9].
A further improvement of energy conversion efficiency to 8 percent or more [10] was achieved with a cell that retained over 98 percent of its initial performance after 1 000 h of accelerated tests including thermal stress at 80C in the dark or 1 000h of visible light soaking at 60C. This cell used a robust electrolyte of low volatility in conjunction with an improved ruthenium dye code-named K-19, grafted together with the co-absorbent, 1-decylphosphonic acid onto the TiO2 film.
Another new member of the same dye, code named Z-910 absorbed light more completely and over a wider spectral range [11]. The light conversion efficiency exceeded 80 percent between 470-620 nm, reaching a maximum of 87 percent at 520 nm. In full sunlight, a DSSC tested had an overall power conversion efficiency greater than 10.2 percent.
Meanwhile, a research team at the National Institute of Advanced Industrial Science and Technology in Ibaraki, Japan, found that simply pretreating the TiO2 with hydrochloric acid was sufficient to significantly improve the energy conversion efficiency of their DSSC to 10.5 percent [12]. This appeared to be due to increased efficiency in light-harvesting, electron injection and/or charge collection. The researchers used another ruthenium complex, the black dye, with absorption extending into the near IR region up to 920nm, which gives a theoretical efficiency of 19.6 percent for the DSSC. There is certainly plenty of room for improvement, and this will happen rapidly.
In another significant development, Solaris Nanosciences, a company in Providence, Rhode Island, made the cell rechargeable [13], thus claiming the lowest manufacturing cost for a long-life photovoltaic system in the world: less than $3 000 compared with current silicon technology outlays of $12 000 or more.
The life-time of the cell is extended by a chemical process that allows the degraded dye in already installed cells to be removed and replaced with a new dye, restoring the performance of the original solar cell.
The recharging process was independently confirmed at the Swiss Federal Institute of Technology Lausanne headed by the inventor Michael Grätzel. “Our evaluation has shown without doubt that the cell performance after three coloration cycles remained intact, and could even be pushed beyond the initial cell output.” He said.
The recharging process has the advantage that the cell could be refilled with new generations of dyes, thus effectively upgrading the solar cell during its life-time, obviating the need for total replacement of the expensive equipment, thereby saving on both the financial and environmental costs involved.
Another advantage of these cells is that they are good for high latitudes. They do not have the reflectivity of inorganic materials such as silicon, which allows them to have greater conversion efficiency when the sun is at high angles relative to the cell.
The Solaris cell is currently running at a conversion efficiency of seven percent, but Dr. Nabil Lawandy, CEO of Solaris Nanosciences, is confident that further improvements are in the pipelines. “We expect the first prototypes to be through the testing cycle in about 12 months and then we will be considering a manufacturing strategy with a target of 1000 panels (20 m2 module) annually for the first manufacturing plant.” He said.
In fact the first commercial products in DSSC have already appeared [7]. Companies like Konarka in the US, Aisin Seiki in Japan, RWE in Germany and Solaronix in Switzerland, are developing new products based on it. Particularly interesting are applications in construction, such as electricity generating glass tiles. The Australian company Sustainable Technologies International has produced such tiles on a large scale for field-testing, and the first building has been equipped with a wall of glass tiles.
Things are moving fast in dye-sensitised and other organic solar cells; fast enough for people to overlook the serious toxicities of some of the main components. There is justification for the opinion that ruthenium dyes, and indeed all ruthenium compounds are “highly toxic” and “carcinogenic” [14]. A study published in 2000 indicated that the N3 ruthenium dye used in the DSSCs is not mutagenic [15], but its other potential toxicities have not been investigated.
Although conventional TiO2 may be relatively harmless, many ultrafine nanoparticles (less than 1 micron), such as those used in DSSCs, are pathogenic [16], and chronic exposure to the nanoparticles may result in fibrosis and airflow obstruction in the respiratory tract [17].
It is important for proponents and developers of these very promising solar cells and applications to ensure that researchers and workers as well as the public are protected from the hazardous materials, that appropriate containment and recycling of wastes take place to prevent environmental pollution, and that research on safety and safe use goes hand in hand with development and commercial exploitation. In addition, effort should be devoted to finding safer alternatives for toxic materials.
Article first published 18/01/06
Comments are now closed for this article