Metabolic disease transmitted through sperm RNAs while mothers pass microbiome diversity to their children Dr. Eva Sirinathsinghji
In recent years there has been accumulating evidence that parental environments can affect the outcome of their offspring. Studies have identified parental diets, lifestyles as well as maternal care, trauma and exposure to toxins, to influence the health and behaviour of their children, some of which persist for multiple generations.
Evidence of natural genetic engineering [see [1] Evolution by Natural Genetic Engineering,SiS63), epigenetic inheritance and the transmission of parental microbiomes are numerous ways in which experiences are passed down the generations. This line of inquiry has major implications for how we understand evolution and most importantly from a medical perspective, how we are able to ensure the health of future generations. Recognising that through maintaining our own health, we give ourselves the potential to secure a healthy life for our children, will likely bring more success than giving into to the fate of our genes and yet to be developed medical interventions to override our genetic ‘faults’.
Lamarckian inheritance, the inheritance of acquired traits, is now firmly back mainstream (see [2] Epigenetic Inheritance through Sperm Cells, the Lamarckian Dimension in Evolution,SiS42), increasingly exposing Neo-Darwinism and the Central Dogma of molecular biology as simplistic ideologies. The reductionism of old is being replaced by a new science that involves information passing back and forth in ways that enables life to adapt much more intelligently than would be possible under natural selection. It means that not only can we take charge of the health of future generations, but also that mistreating our health and the environment can have much longer lasting effects than expected. Many environmental chemicals, including pesticides and the household plastics product bisphenol A (BPA) (see [3] Epigenetic Toxicity, SiS 41) are well known to have transgenerational effects. Similarly, diets can also have transgenerational effects, as described below. This is of increasing concern with diet related diseases rising globally. It serves as a further warning to people everywhere who are being increasingly exposed to Westernised processed foods high in sugars, fats, and chemicals and low in fibres and nutritional content.
Non-genetic inheritance through the male germline has already been shown in both invertebrates and mammals. In humans for example, epidemiological data show that malnutrition in fathers can alter the health prospects of children later in life. Much of this has been found to be mediated through non-coding RNAs (ncRNA) that go on to influence epigenetic control of gene expression in the genome of offspring, with effects lasting many generations (see [4] RNA Inheritance of Acquired Characters, SiS 63). However, there are around 50 known ncRNA species and the extent to which individual species are involved remains unclear. A new study published in Science is the first to implicate sperm transfer RNA (tRNA)-derived small RNAs (tsRNAs) in this phenomenon, complimented by another study in the same issue elucidating the mechanisms of tRNA biogenesis and their regulation of gene expression in offspring embryos.
The first study, led by researchers in both China and the US found that a high fat (60 %) diet fed to male mice resulted in offspring that by 7 weeks of age suffered impaired glucose and insulin tolerance [5]. To find out what might be responsible for transmitting the information from father to child, they first purified total RNAs from sperm of mice fed both high-fat and control diets and injected them into zygotes. This experiment recapitulated the impaired glucose tolerance, though not impaired insulin tolerance in the offspring, suggesting that additional factors such as DNA methylation in addition to ncRNAs are required for the full inheritance of the metabolic traits. The researchers performed global RNA sequencing to see if there were any changes in the expression of particular types of RNA species. They found that the sperm of mice on the high-fat diet were enriched for a subset of small RNAs that are 30-34 nucleotides (nt) long, deriving from one end (5’) of transfer RNAs, termed tRNA derived small RNAs (tsRNAs). Injection of just this form of tsRNA into zygotes again resulted in offspring with impaired glucose tolerance and mild or no insulin intolerance. Analysis of the sperm tsRNAs revealed a total of 10 types of modification, 2 of which were enriched in the sperm of mice on the high-fat diet. The significance of that is still unknown, but suggests the modifications play a role in RNA stability and/or inheritance.
The researchers then looked at global gene expression in islet cells in the pancreas of the offspring to unravel the cause of the inherited metabolic disease. They found abnormal expression of genes involved in metabolic pathways (including ketone, carbohydrate and monosaccharaide metabolisms) in offspring of males fed the high-fat diets. There were also differences in the methylation patterns (chemical tags on DNA that mediate gene expression) of 28 genes, though none of these matched the dysregulated genes, suggesting that the changes in methylation patterns were not directly related to the changes in transcriptional activity. Injecting the tsRNAs into zygotes identified that it was indeed the tsRNAs themselves that were responsible for the changes in gene expression, with their injection causing gene expression changes in metabolic regulation pathways and others including protein transport. The tsRNA sequences matched the gene promoter regions of many of the dysregulated genes, suggesting that the tsRNAs themselves are directly responsible for the perturbed gene expression.
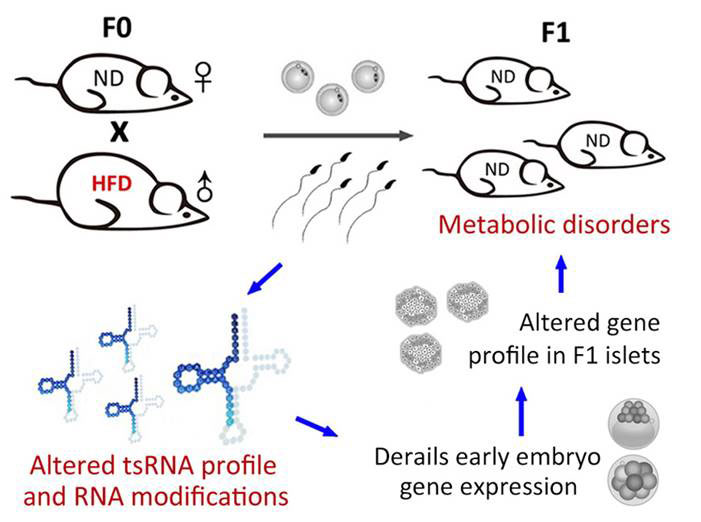
Figure Mechanism of tsRNA-mediated inheritance of metabolic disorder from father to offspring [5]
The second study found a similar pattern of inheritance, but this time male fathers were fed a low protein (10 %) diet versus a control (16% protein) diet, resulting in metabolic changes in the offspring including increased expression of the hepatic cholesterol biosynthesis gene squalene epoxidase [6]. Sperm of low protein-diet males had an increased abundance of certain tsRNA species (~28-34nt 5’ tsRNAs).
Looking in embryonic mouse stem cells, the researchers used antisense RNAs to bind and thus block expression of the tsRNAs. They found that blocking expression of one particular type (tsRNA-Gly-GCC) resulted in dramatic upregulation of ~70 genes. These genes are highly expressed in pre-implantation embryos and are regulated by a type of mouse endogenous retroviral elements, MERV-L. Endogenous retroviral elements constitute a large chunk of mammalian genomes (~10 % in humans) and were once thought to be redundant parts of the genomes that originated from ancient viral infections; but in recent years shown to be functional and dysregulation of their expression may well contribute to a variety of illnesses (see [7] Ancient Viral Genes in Human Genome Reactivated in Human Disease, SiS 69). Injecting antisense oligonucleotides also blocked expression of MERV-L itself.
Two-cell stage embryos were also injected with total RNA purified from male sperm on low-protein diets, which resulted in alterations in gene targets of tsRNA-Gly-GCC. Further, microinjection of tsRNA-Gly-GCC alone led to downregulation of MERV-L target genes. The biogenesis of the tsRNA fragments was found to occur in epididysomes, small vesicles that fuse with sperm during transit through the epididymis, rather than during spermatogenesis in the testes. Contained within these vesicles, the RNA was resistant to degradation and was even present in a transgenic mouse strain that is spermless, showing that they do not originate inside sperm but instead are introduced to sperm as they mature and are increasingly abundant as the maturation goes on.
The human microbiome has received increasing attention in its role in human health and disease (see [8] The Forgotten Organ – The Human Microbiota, SiS 61 [9] How Microbes Influence our Minds, SiS 61 [10] The Gut Microbiome and Cancer, SiS 62) and is another mechanism whereby health status can be passed from parents to offspring. A new study shows that female mice fed a low-fibre Western diet leads to reduced diversity of the gut microbiome that is inherited by offspring [11]. Mice fed normal diets for the first 6 weeks of their life were then put on either a low- or high-fibre diet for another 7 weeks. Loss of microbial diversity was observed under the low-fibre diet (60 % of taxa were less abundant), and if mice were put back onto the control diet the diversity did not fully return (30 % of taxa were less abundant) after 6 weeks of the high-fibre diet. What was most striking was that this effect was magnified over 4 generations. For each generation, switching them back to a high fibre diet led to a small return of microbial diversity, but most did not return, with 141 out of 208 taxa being undetectable in the fourth generation. Even a switch to a high fibre diet was not sufficient to increase diversity of gut microbes, leaving faecal transplants as a last option for restoring the microbial community after generations of low fibre foods. The researchers tested the efficacy of this approach and found that it led to full restoration of gut diversity. These results led to the conclusion that the consumption of Western diets may be responsible for the reduced diversity of gut microbes seen in the West compared to other regions, suggesting that it may be necessary to ‘re-wild’ the gut of the Westernised world.
The updated theories of Lamarckism and the inheritance of acquired traits has many implications for the way we view the world including resisting the scientific hegemony that supports a corporatized food and health system.
International food programmes have traditionally focused on food security and micronutrient deficiency, but the diet related health burdens due to non-communicable chronic diseases are now surpassing those due to undernutrition in most parts of the world [12], a trend related to convergence to less healthy diets globally. Promotion of traditional healthy diets and dietary education needs to be encouraged, while the corporate takeover of food including policies that erode food sovereignty and local food systems as well as transnational marketing and investment of unhealthy foods needs to be resisted.
Article first published 08/02/16
Got something to say about this page? Comment