Structured water is among the hottest topic in science but no one has seen it until now Dr. Mae-Wan Ho
Structured water is serious science. It must be real because generations of big instruments have been deployed to detect it; the latest to capture the headlines being ultra-fast electron crystallography [1, 2] after more than a decade of neutron scattering, X-ray diffraction, nuclear magnetic resonance, etc., not to mention the considerable number-crunching to extract information out of the data, and hours upon hours of computer simulations that go into modelling it.
But no one has actually seen structured water itself, which makes it much more elusive than unidentified flying objects or poltergeists.
The best that the string of high-power instruments has produced are ghostly diffraction patterns, and bumps on squiggly-lined spectra that only the specialists running these scientific séances can decipher. Nevertheless, structured water has captured the public imagination; and enterprising companies have made it a selling point for water-purification devices, or in one case, for bottled water selling at £1.50 a time (though I am told it has gone off the market since).
But structured water, or at least, one form of it, has finally been caught on camera; not an ordinary camera, admittedly.
Researchers at the A.J. Drexel Nanotechnology Institute in Drexel University, Philadelphia and University of Illinois at Chicago, USA, and the Tokyo Institute of Technology, Japan, have produced stunning high-resolution transmission electron-micrographs of carbon nanotubes of different sizes with water trapped inside them [3, 4].
Carbon nanotubes, a new form of carbon discovered in 1991, are long thin tubes that are either single-walled or multi-walled, and can be closed or open at the ends, depending on how they are made. Yury Gogotsi, Haihui Ye and coworkers have previously found that autoclaving multi-walled nanotubes with closed ends and inner diameters ranging from 200 nm to 2 nm caused water to enter through defects in the walls and remain trapped inside the hollow tube. This provides a great opportunity for investigating the properties of water at different scales of confinement. There has been a spate of discoveries suggesting that water and other fluids in confined spaces (nanometre dimension) have weird properties. They tend to show new phases and behaviour other than the usual ones they show in bulk.
In the case of water, theoretical studies have predicted new phases of ice inside carbon nanotubes, but not all scientists agree. So there is nothing like real data to settle the issue.
The nanotubes are sealed with water in a gold capsule, which is then treated in the autoclave at 300 – 650C under 20 to 80 MPa pressure (1 MPa, MegaPascal = 1 000 000 Pascals ~ 10 atmospheres). This treatment causes the nanotubes to fill up with water, and ready to have their pictures taken by a high-resolution transmission electron microscope (TEM). Accompanying the high-resolution imaging is a parallel modelling of the molecular arrangements using a computer software package, HyperChem. As a result, corresponding HyperChem snapshots are obtained, showing models of water molecules inside nanotubes of diameters equal to the tubes being observed under the electron microscope.
The researchers discovered that in large diameter carbon nanotubes (10 to 200 nm), water behaves fairly conventionally, much as they would in an ordinary capillary tube. The liquid water inside the hollow tube shows up in low contrast, and at the boundary between liquid and gas phases, a typical meniscus (concave surface) is observed (Fig. 1a). Such a curvature indicates strong interaction between water molecules and the inner wall of the nanotube. And as typical of liquid water when heated up by successive electron beams from the electron microscope, the volume shrinks and finally disappears as all the water evaporates into the gas phase. But as the water is trapped, this gaseous water eventually condenses again into liquid, which provides a method for transporting water with the nanotube. During the evaporation process, the water/nanotube interaction can be significantly accelerated by rapidly converging the electron beam at the water, so that a hole can be created in the wall of the nanotube at that point as the water molecules get burnt off, carrying the bit of wall with them. This, the researchers observe, is a very good way of drilling a hole in a nanotube for nanotube-engineering.
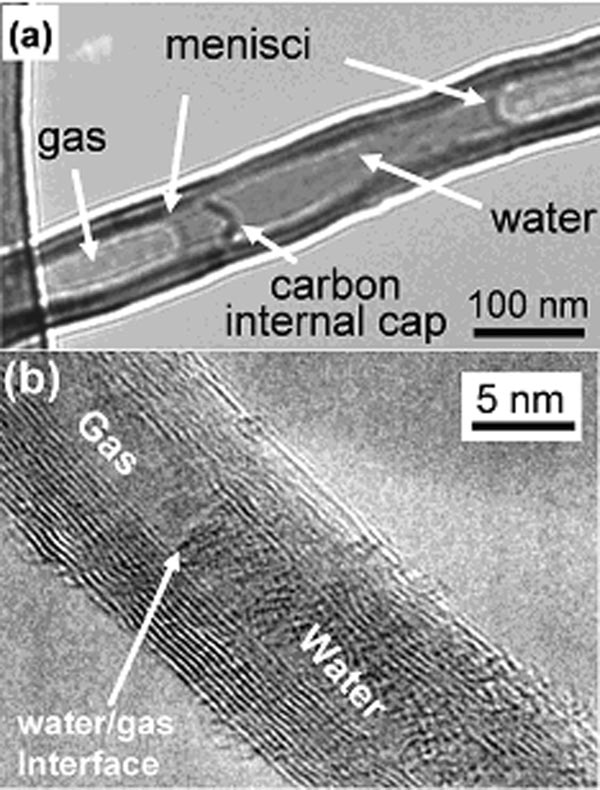
Figure 1. High resolution TEM of big nanotube (a) and small nanotube (b) with water trapped inside
Pictures of small diameter nanotubes (2 to 5 nm) are something else. The liquid water shows up in high contrast, giving a bright beady appearance, quite unlike the water trapped in the bigger nanotubes (Fig. 1b). Also contrary to the pictures seen in large nanotubes, there is no meniscus separating the liquid from the gas phase. In fact, the water molecules appear not to interact with the wall of the nanotube at all, but are concentrating their interactions with one another, leaving a typical gap between the liquid water and the wall.
And now comes the bit of science that convinces someone like me that the bright and beady appearance of the water is really structured water. The researchers turned on the HyperChem to simulate the molecular arrangements, then use the molecular arrangements to back simulate what it would look like under the electron microscope at different degree of focus. And lo and behold, we get the bright, beady appearance, in which the ‘beads' themselves can be identified with clusters of a few molecules of water (Fig. 2).
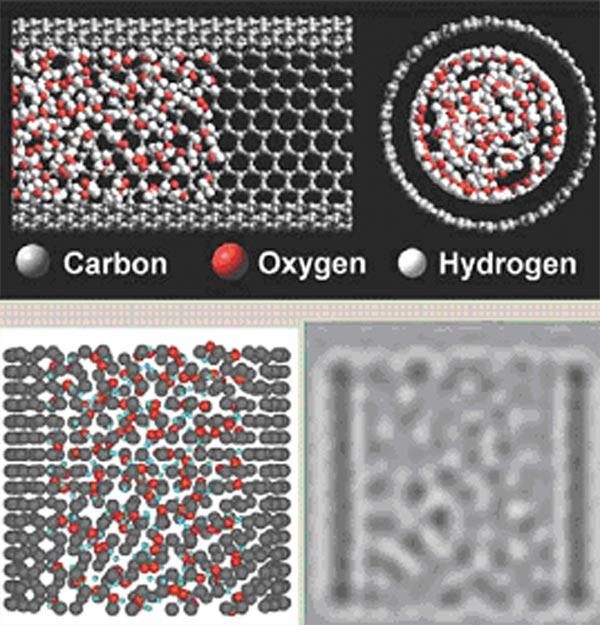
Figure 2. Hyperchem simulation of water molecules inside 4n diameter nanotube (top), and corresponding
TEM simulation in longitudinal section (bottom right)
What this means is that the individual water molecules are sufficiently ordered (structured) and restricted in motion to be captured on film. This structured water is a cylindrical lattice-work some ten layers of molecules across for a 4 nm diameter nanotube. In some pictures, like the nanotube filled with heavy water (made with deuterium, a heavy isotope of hydrogen) (Fig. 3), the liquid water phase inside the small nanotube looks rather like a twisted multi-strand pearl necklace, suggesting the formation of strings of hydrogen-bonded water molecules.
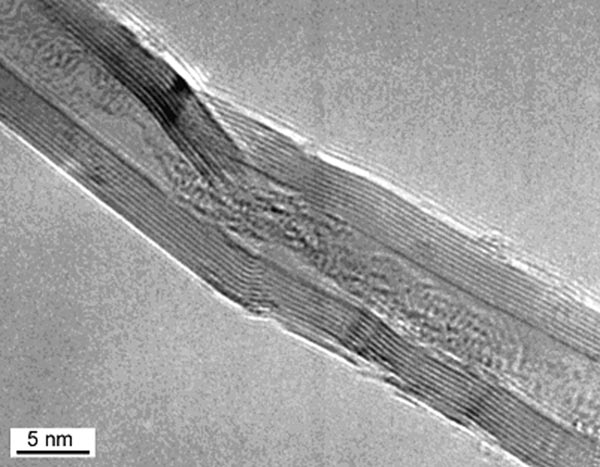
Figure 3. Nanotube with heavy water
This research raises a host of interesting questions, including what kind of proton-conduction properties such water cylinders would have (“Positive electricity zaps through water chains”, this series). Perhaps something like a super-conducting proton-cable, I imagine, with lots of energy-efficient applications, no doubt…
Article first published 02/11/05
Got something to say about this page? Comment