100% Renewables
High power and energy density devices with applications for electric vehicles, grid renewables, and yet to come: foldable displays and a host of portable, wearable consumer electronics Dr. Mae-Wan Ho

Capacitors are energy storage devices that store energy electrostatically as separated positive and negative charges. Supercapacitors store 10 to 100 times more energy per unit volume or mass (energy density) than ordinary capacitors, and can also accept or deliver the 10 to 100 times more energy per unit time (power density) than most batteries. In addition, they can withstand tens of thousands or more charge/discharge cycles without losing their original energy storage capacity, whereas batteries typically degrade substantially after hundreds of charge/discharge cycles [1, 2]. Supercapacitors are widely seen as bridging the energy gap between traditional capacitors with high power output and batteries with high energy storage capacity. But they are extremely versatile and support a broad range of applications, from consumer electronics, to renewable energy storage systems to transport vehicles, and will replace traditional capacitors altogether as well as batteries in certain applications.
In November 2010, a report from Nanomarkets predicted that the fastest growing market for supercapacitors in consumer electronics, expected to exceed $725 million by 2016; that frequency regulation in next-generation electricity grids will provide $540 million business for supercapacitor firms in 2016; while the transport/vehicles sector’s market share of 60 % will fall to around 35 % as new applications for supercapacitors emerge [3].
Energy storage in supercapacitors depends on electrical double-layer capacitance (EDLC) and pseudoapacitance [2]. EDLC is energy stored in separated positive and negative charged ions at the interface between the surface of a conductive electrode and an electrolyte without electrochemical reactions occurring. In contrast, pseudocapacitance is energy stored as the result of fast and reversible electrochemical reactions occurring at or near the surface of the electrode; hence supercapacitors dependent on pseudocapacitance are also known as electrochemical supercapacitors (ESs). ESs with capacitance up to 10 000 F (Farad) at 1.2 V are currently available. But they are 10 times larger than conventional batteries for a given charge [1].
Energy storage by EDLC strongly depends on the available electrode surface area for ion adsorption and the conductivity and pore structure of electrodes, which affect the transport of electrons and ions. That is why most electrodes are fabricated from carbon materials with high surface area such as activated carbon, mesoporous carbon, carbon nanotubes and in particular, the most recently discovered carbon allotrope (different structural form of an element) graphene (see [4] Graphene Micro-Supercapacitors for On-Chip Energy and other article in the series, SiS 59). However, most electrodes in commercially available supercapacitors are made from activated carbon, and few the recent laboratory successes with the new forms of mesoporous carbon have yet been translated into commercial products.
Carbon-based EDL supercapacitors can deliver high power density and have excellent cycling stability, but they have low energy density on account of limited specific capacitance of carbon materials; though an appropriate combination of carbon isomers and electrolyte can overcome this limitation, as recent research demonstrates (see below).
Pseudocapacitive materials such as transition metal oxides and hydroxides (RuO3, MnO2, Ni(OH)2, Co(OH)2, NiO and Co3O4, nitrides, carbides and conducting polymers like polyaniline, polypyrolle, and polythiophene, provide much higher specific capacitance, up to 10-fold; though most of them have poor rate capability and low electrical conductivity. A common strategy is to use composites of pesudocapacitive materials and carbon for electrodes [5].
Hybrid supercapacitors use one electrode with mostly electrostatic capacitance and the other mostly electrochemical capacitance, such as the lithium-ion capacitor [6]. Activated carbon is used as cathode, and the anode consists of carbon material doped with lithium ion. This doping lowers the potential of the anode and allows a relative high voltage output (2.2-3.8V) compared with other supercapacitors.
The energy density vs power density of various capacitors and batteries are given in so-called Ragone charts (Figure 1). As can be seen new developments in supercapacitors are driving up both energy density and power density, with the additional benefits of minimum maintenance and durability.
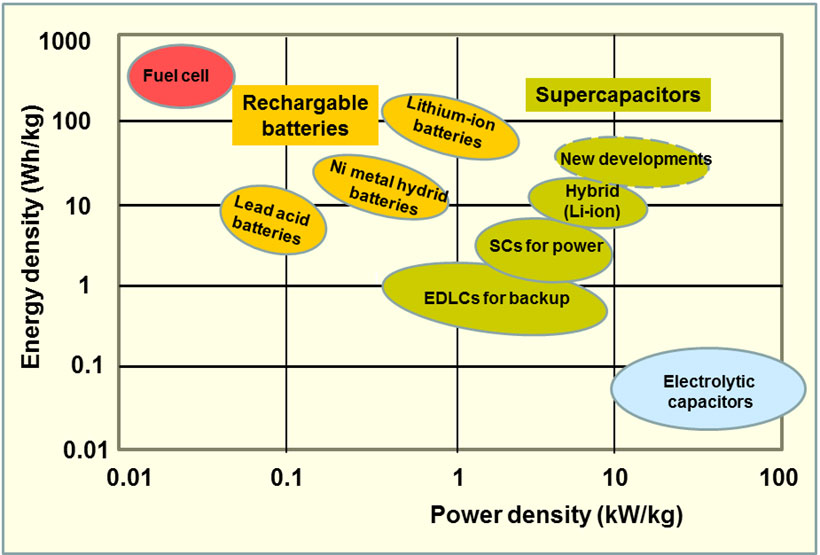
Figure 1 Ragone chart of energy density vs power density of different energy storage devices
Transport
The ES patent was filed in 1957, though it was not until the 1990s that ES began to attract attention for applications in hybrid electric vehicles. Its main function is to boost the power supply for acceleration and to recover the energy from braking. Many successful applications have been documented (see [1]).
One important area is in city transport. Supercapacitors not only reduce energy consumption, but can also do away with catenary overhead lines for trams and light-rails in historic cities. In 2003, Mannheim adopted a prototype light-rail vehicle (LRV) that uses the MITRAC energy saving system from Bombadier Transportation to store mechanical braking energy with a roof-mounted supercapacitor system of 518 V with energy content of 1.5 kWh. It can drive up to 1 km without catenary lines. Compared with conventional light rail vehicles that return energy into the grid, onboard energy storage saves 30 % and reduces peak demand by up to 50 %. In 2009, supercapacitors enabled LRVs to operate in historical precincts of Heidelberg, Germany, without catenary overhead wires. The supercapacitors are charged at stop-over stations when the vehicle is at a scheduled stop. A further 11 units were ordered in 2011. Also in 2009, a tram in Paris operates with a roof-top energy recovery system STEEM manufactured by Alstom consisting of 48 supercapacitors to store braking energy. The system provides the tram with a high level of energy autonomy by enabling them to run without catenary power on parts of its route, recharging while traveling on powered stopover stations. During tests, the tramset used an average of ~16% less energy.
2012 was a bumper year for supercapacitor transport. Tram operator Geneva Public Transport began testing an LRV equipped with a prototype roof-mounted supercapacitor unit to recover braking energy. Siemens began delivering supercapacitor enhanced light-rail transport systems that include mobile storage. Hong Kong’ South Island metro line is to be equipped with two 2 MW energy storage units expected to reduce energy consumption by 10 %. And China’s CSR Zhuzhou Electric Locomotive corporation presented a prototype two-car light metro train with a roof-mounted supercapacitor that can travel up to 2 km without wires, recharging in 30 seconds at stations via a ground mounted pickup. The supplier claims the trains could be used in 100 small and medium-sized Chinese cities. In Lyon, France, the SYTRAL (Lyon public transportation administration) started experiments of a “way side regeneration” system built by Adetel Group, which has developed an energy saver named “NeoGreen” for LRV, LRT (light rail trams) and metros. Seven street cars powered by supercapacitors were scheduled to go into operation in 2014 in Guangzhou, China. The supercapacitors are recharged in 30 seconds by a device positioned between the rails, and powers the tram for up to 4 kilometres.
Similar successes have been achieved for city buses all over the world. In 2005, Shanghai tested a new electric bus, ‘capabus’ that runs without powerlines using large onboard supercapacitors that partially recharge whenever the bus is at a stop (under ‘electric umbrellas’), and fully charged up in the terminus. In 2006, two commercial bus routes began to use the capabus. It was estimated that the supercapacitor bus was cheaper than a lithium-ion battery bus, and one of its buses had one-tenth the energy cost of a diesel bus, with a lifetime fuel savings of $200 000. A hybrid electric bus, tribrid, was unveiled in 2008 by the University of Glamorgan in Wales for use in student transport. It is powered by hydrogen fuel or solar cells, batteries and supercapacitors.
Supercapacitors have made substantial inroads into the glamorous world of motor-racing (see [1]). The Toyota TS030 Hybrid LMP1 uses a hybrid drivetrain with supercapacitors, and won three of the 8 races in the 2012 FIA World Endurance Championship season.
Electric and hybrid electric vehicles using supercapacitor/battery combinations are well investigated, and a reduction in fuel consumption by 20 to 60 % has been claimed by recovering brake energy. The short charging time, stable electrical properties, broad temperature range and longer lifetimes offered by supercapacitors are obvious advantages, though mitigated by the weight, volume and cost. There is obviously much room for improvement in increasing energy and power density, and reducing weight, volume and cost.
Renewable energy storage
The rapid rise of renewable energy and its distributed generation has led to a radical transformation of the energy grid (see [7] Distributed Grid Energy Storage Comes of Age with Renewables, SiS 65). In particular, grid energy storage at all levels has become an important asset, with batteries figuring most prominently. The next breakthrough in grid capacity may be supercapacitors. While batteries produce and store more energy per unit volume, they cannot deliver it in large amounts per unit time. That’s why they cannot supply enough power to meet peak demands, and it takes hours to recharge from a battery. Supercapacities can deliver (and accept) a great deal of energy in a very short time, seconds or less. Furthermore, they have a wide operating temperature range, and are robust to charging and discharging hundreds of thousands of times. Supercapacitors can therefore prolong battery life.
Supercapacitors tied to solar and wind would make the power output much more reliable, said Dr Kimberly McGrath, Director of Business Development at San Diego-based Maxwell Technologies [8].
A supercapacitor’s energy capacity depends crucially on the surface area and conductivity of the electrode material. Commercially available supercapacitors contain electrodes mostly made from activated carbon and do not give good performance when tested in the lab [9]. Meanwhile laboratory research over the past two decades has identified a whole range of carbon isomers synthesized under different conditions that are far superior to activated carbon in both electrical capacity and conductivity.
For example, a team of researchers led by Wu Lu at Gwangju Institute of Science and Technology, Republic of Korea, fabricated supercapacitors using as electrodes graphene powder synthesized from graphite powder pre-oxidized into graphene oxide and then reduced back to graphene after extensive washing and tip sonication to exfoliate the suspension completely [10]. For the electrolyte, they used the ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIBF) with superior capacitance and an operating voltage of 3.5 V. The cells created delivered an energy density of 64.18 Wh/kg, a power density of 8.75 kW/kg (see Fig. 1 for how it compares favourably with batteries and existing supercapacitors), and a specific capacitance of 150.9 F/g at a current density of 5 A/g. The discharge time was 25 s at 5 A/g. The power and current densities were the highest reported as of 2013. The supercapacitors remained stable after several tens of thousands charge/discharge cycles at high current. Above all, the authors stated, the method of constructing the electrode could be up-scaled to industrial manufacture, where the slurry of graphene powder and other active materials can be applied uniformly to stainless steel mesh and rolled into a cylindrical shape. Later, we shall be looking at other examples of ongoing research on materials and design that could improve the capacity and reduce the weight and bulk of supercapacitors, as well as the cost of manufacture.
Consumer Electronics
The market for batteries and supercapacitors in consumer electronics is forecast to be worth US$86 billion by 2023 [11]. This includes laptops phones, tablets, cameras and wireless sensors, not to mention a whole new range of wearable electronic monitors and flexible displays, robotics, toys, etc., limited only by the imagination.
As in stationary applications, supercapacitors can extend battery life in portable electronics [12]. The company cap-XX based in New South Wales, Australia, makes thin supercapacitors for incorporation into portable electronics such as mobile phones, digital cameras, digital music players, toys and ebooks [13].
Another company Paper Battery Co based in Troy, New York, USA [14] offers even thinner (<<0.5 mm for 4.5 V device) for consumer electronics.
The current market for consumer supercapacitors is still small compared to batteries. As of 2013, portable speakers powered by supercapacitors have been available [15].The only other commercially available consumer product is a graphene supercapacitor that can extend the battery life of smartphones [16]. The graphene is made by chemical vapour deposition licensed from the Materials Science group at Oxford University in the UK. The supercapacitor charger recharges the phone in 5 minutes. It is by no means a micro-supercapacitor, as it weighs 12.35 ounces, nor is it cheap, retailing for US$149.
However, there is an amazing range of micro-supercapacitors constructed in laboratories around the world, some paper thin that can be used on-chip, or on flexible substrates that could be bent or folded without impairing operation, and a whole category of fibre supercapacitors that could be woven into textiles for clothing, displays, for artificial skin of robots, etc. (see [17] Next Generation Supercapacitors Coming, SiS 66)
Article first published 23/03/15
Got something to say about this page? Comment
There are 2 comments on this article so far. Add your comment above.
Todd Millions Comment left 27th March 2015 03:03:18
There were reports of a super capacitor bus on a park loop in Moscow in 2002-the company was showing on search engines for years after-train startup capacitors were offered by them. On stand alone electrical systems with wind/solar power, a 'overnight'24hour capacity that lasts many more cyles than the existing lead acid-can be more expensive-yet reduce the life cycle costs of the whole storage system considerably. This would be a variable saving that would have too be matched carefully,but taking the overnight drain off the days long storage-could easily double the life of the storage system overall.
Alex Comment left 20th September 2015 19:07:49
Hi,
I need more information about Super Capacitor Electric Buses. Who are the manufacturers. What is the advantage of Super Capacitors buses compare with Lithium Ion Batteries.