A hazardous transgenic technology for male sterility originally designed to enforce corporate patent on seeds is now transmogrified into a means for increasing yield, in theory, through enforced outbreeding Dr. Mae-Wan Ho
Scientists led by Deepak Pental at Delhi University are reported to have completed final trials of a genetically modified (GM) mustard, and will submit a report to the government in the hope of overcoming stiff opposition to make it the country’s first commercial transgenic food crop [1].
Among the biggest critics of GM crops is Bharatiya Kisan Sangh, a powerful farmers group close to Prime Minister Narendra Modi’s Bharatiya Janata Party (BJP), and it wants the government to stop all field trials.
But Modi believes that allowing GM crops is critical to boosting farm productivity in India, with population reaching 1.5 billion by 2030, overtaking China.
India imports about 60 % of its edible oil at an annual cost of up to $10 billion, its top import after crude oil and gold.
The new GM mustard could substantially reduce this import bill, as according to Deepak, its yield is 26-34 % higher than the national average. He also claimed that biosafety studies did not show any adverse allergenic, toxic or environmental impact. However, there are no scientific papers to back up his claims.
In August 2014, the Modi government resumed field trials for selected GM crops with little publicity [1], and in January, Maharashtra state led by the BJP gave the all clear to trials of rice, chickpeas, corn and aubergine, as well as new varieties of cotton.
So, what is the GM mustard that’s supposed to increase oilseed yield and save India billions of dollars on edible oil imports? It is genetically modified to be male-sterile, i.e., unable to produce pollen, using a technology originally designed to enforce corporate patent on seeds, because the seeds will not set unless it is pollinated by another non male-sterile variety (see Box for details of the original technology). It has now been transmogrified into a means for increasing yield, in theory, through enforced outbreeding and heterosis [2] or hybrid vigour, the enhancement of yield and other characteristics in the hybrid compared to the inbred parental lines.
(modified from [3] Killing Fields near You, Terminator Crops at Large, I-SIS News 7/8)
Male-sterile seeds are engineered by means of ‘terminator technology’, so named by critics because it renders harvested seeds sterile in order to enforce corporate patents on GM seeds.
The terminator system requires two components, the enzyme barnase, which kills the germ cells, and a ‘site-specific’ recombinase, which is used to block the action of barnase until it is required. There have been 132 field trials of crops with barnase by 2000, the vast majority without risk assessment. Crops modified for male sterility include rapeseed, corn, tobacco, cotton, Brassica oleracea, potato, poplar, chicory, petunia and lettuce. The USDA commercial release data include 4 crops with barnase: a corn and a canola by AgrEvo, a chicory by Bejo, and another corn by Plant Genetic Systems. Separately, the ‘site-specific’ recombinase has been engineered into corn and papaya, and there have been 14 field trials between 1994 and 1998, without environmental impact assessment, as it was deemed unnecessary.
There are more than 150 US patents listing barnase or site-specific recombinase or both by 2000[3] the oldest going back to 1987. The first terminator patents to catch public attention were those jointly owned by US Department of Agriculture and Delta and Pine Land Company, which Monsanto had intended to acquire. The novelty in those patents is the proposal to combine the barnase with the site-specific recombinase, giving the company complete control over the hybrids as well as proprietary chemicals that control gene expression.
The first component barnase is literally the ‘terminator’, it is an enzyme that breaks down RNA. RNA is an intermediate in the expression of all genes, (and many RNAs that do not code for genes are now known to have vital regulatory functions, see [4] Non-Coding RNA and Evolution of Complexity, SiS 63) and that is why barnase is lethal to all cells in which it is expressed. Barnase is specifically inhibited by barstar. Both barnase and barstar are produced by a soil bacterium, Bacillus amyloliquefaciens. Inside the bacterial cell, barstar binds to barnase in a one-to-one complex, disarming the latter so it can do no harm. However, when barnase is secreted outside, it is no longer bound to barstar and is thus harmful to other cells.
The second component ‘site-specific’ recombinase is an enzyme that recognizes specific ‘sites’, or short DNA sequences, labelled ‘s’ in the diagram below. Any stretch of DNA sequence flanked by two such sites will be spliced out by the recombinase.

To engineer pollen sterility, the barnase gene is placed under the control of a promoter, a gene switch that allows the gene to be expressed, and in this case only in cells that develop into anthers the male part of the flower. The barnase with its anther-specific promoter is stitched next to the transgene of interest, say, a gene coding for herbicide tolerance, also with its own promoter. Theoretically, there will be no fertile pollen from this transgenic crop. In the case of crops that are normally self-fertilised, there will be no seeds set. In out-crossing plants, the only fertile seeds set will be those fertilised by non-GM varieties nearby, which will not be herbicide tolerant; so farmers who want the herbicide tolerant trait will have to buy fresh seeds from the company every season.
The problem is that such a male-sterile line by itself cannot be propagated, and if fertilised by another male fertile strain, it no longer breeds true. To propagate the line, the ‘site-specific’ recombinase can be used. For example, the promoter of the barnase could be blocked by a blocking sequence flanked by sites recognised by the recombinase.

The recombinase can be engineered into the same transgenic line with the barnase gene for male sterility. The recombinase is placed under the control of a promoter that responds to an external chemical, say, the antibiotic tetracycline.
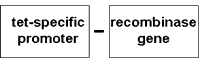
When tetracycline is applied, the recombinase is expressed, and splices out the blocking sequence in the barnase promoter, so barnase is expressed. By treating harvested seed with tetracycline before they are sold to the farmer, the company can ensure that the plants grown from the seeds will be pollen sterile.
If female-sterility is required, the barnase gene could be placed under the control of a promoter that works in cells that develop into ovules, the female part of the flower, and the rest is similar.
Alternatively, the recombinase may be engineered into a GM line with the gene coding for barstar, which, when crossed with the male sterile GM line containing barnase, will produce a hybrid. The hybrid, treated with tetracycline, will produce plants that will still set seed, at least in theory, because the barstar inactivates the barnase.
Later versions of terminator crops make use only of barnase and barstar in separate transgenic strains that when crossed restores fertility (see main text).
According to Pental and colleagues, B. juncea is a predominantly self-pollinating crop [2]; but this is untrue, only the commercial varieties have been inbred from originally freely outbreeding varieties [5]. They reasoned that to produce hybrids require the introduction of male sterility into a strain that could act as a female parent in the F1 hybrid (that gives enhanced productivity), and a suitable restorer of fertility that when crossed with the male-sterile transgenic strain, produces seeds that are fertile, and on self-fertilization, regenerate seeds with the male sterile trait [2].
They used the barnase-barstar system without recombinase (see Box). The barnase strain contains besides the barnase gene (driven by the tapetum (anther)-specific promoter TA29), the bar gene for resistance to the herbicide glufosinate driven by the cauliflower mosaic virus (CaMV) 35S promoter, which is notorious for instability and for causing unregulated expression of other genes [6]. Pental’s team improved the stability of the strain by using a spacer DNA fragment as insulator to prevent deregulated expression of the barnase gene by the CaMV 35S promoter [7]. The barstar strain contains besides the barstar gene driven by the tapetum-specific promoter TA29 or A9, a hydromycin phosphotransferase (antibiotic resistance) gene driven by the CaMV 35S promoter. The barstar gene was also improved by changing its base sequence (without changing amino acid sequence of the protein encoded) in order to optimize for expression in plants, and a combination of wild-type and modified barstar driven by TA29 and A9 promoter respectively was shown to be efficacious in restoring fertility [8]. In yet another improvement, the barnase transgenic plant was retransformed with the improved barstar construct, resulting in high frequencies of double transformants (with both barnase and barstar genes), which after selfing, produced a considerable number of restorer transgenic plants that have the same genotype as the barnase transgenic line [9].
As mentioned earlier, there is no evidence that the transgenic mustard can increase yield when crossed to a male-fertile variety, which was the stated purpose for creating the male-sterile transgenic strain in the first place. B. juncea is not naturally a self-fertilizing species, and inbred strains have been created from originally outbreeding varieties [5]. There are no published scientific papers or publicly available reports that present evidence of increased yields by crossing the transgenic mustard strain with a male-fertile variety.
There have been no published scientific papers or publicly available reports on biosafety studies that addresses the following issues.
Barnase is an extremely potent cell poison
Barnase is an enzyme that breaks down RNA indiscriminately, and known to be an extremely potent cell poison. Traces of barnase are toxic to the rat kidney [10] and to human cell lines [11]. Barnase is actually being exploited as a conditional ‘suicide gene’ to cause cell death in mammalian [12] and human [13] cells when it is induced. It is also toxic to insect cells [14] as well as plant cells in which it is expressed. In the transgenic mustard, the toxic gene is placed under the control of a promoter only active in tapetal cells that give rise to pollen. However, when the plant is ingested, the gene (present in all plant cells) can transfer horizontally to the animal/insect cells and become expressed, with potentially fatal consequences. There have been no studies on horizontal transfer of the transgene, which is a distinct possibility based on recent evidence (see below).
Gene flow is real
The possibility of gene flow via pollen transfer is real (see [3]). ‘Leakiness’ of genetic constructs are well known. A small proportion of the male-sterile transgenic plant may nevertheless produce pollen. Gene escape can occur in particular during crosses with ‘restorer’ barstar transgenic lines. The male sterile line is maintained in a ‘hemizygous’ state with only one copy of the male sterility gene, barnase, linked to a glufosinate-resistance gene, bar. The barstar transgenic plants are homozygous, with two copies on a different chromosome from that containing barnase, each linked to the gene for hydromycin antibiotic resistance. When crossed, the male-sterile line is the female parent, and the offspring (fully fertile) consists of half with one copy each of barnase and barstar gene and is glufosinate resistant, and half with only one copy of the barstar gene, which can be killed off by spraying with glufosinate. The fully fertile plants with both barnase and barstar will produce four kinds of pollen in equal proportions ¼ with both barnase and barstar, ¼ with barnase only, ¼ with barstar only, and ¼ with neither. Thus, half of the pollen grains will have the barstar gene (see Figure 1).
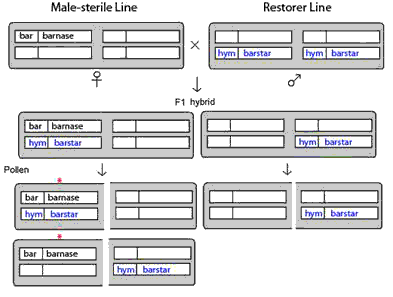
Figure 1 Crossing male-sterile barnase line with restorer barstar line generate F1 hybrids that are fertile and produce pollen with barnase gene, that can fertilize non-transgenic plants
Horizontal gene transfer is highly likely
Since transgenic crops were first commercially released, I have raised the possibility of unintended horizontal spread of transgenes through transgenic DNA (or RNA) being taken up by cells of all species of animals and microorganisms interacting with the plant [15] (Genetic Engineering Dream or Nightmare, ISIS publication). In the intervening years, evidence for the spread of transgenes by horizontal gene transfer has accumulated, but denial and suppression continues (see [16] Horizontal Transfer of GM DNA Widespread, SiS 63). One particular route for horizontal gene transfer to microorganisms in the soil and on the surfaces of plants is via the Agrobacterium and binary vector system used in creating transgenic plants, including the Indian transgenic mustard discussed here [2]. It appears that the Agrobacterium and binary vector can remain in the transgenic plant even after treatment with high concentrations of antibiotics, greatly facilitating horizontal gene transfer [17]; and who knows what new pathogens would be created from the transfer of the barnase gene. Not only that, new research shows that DNA fragments derived from meals, large enough to carry complete genes, can escape digestion in the gut and enter the blood stream to be taken up by cells, and so can RNA (see [18] Nucleic Acid Invaders from Food Confirmed, SiS63). The uptake of the barnase gene and/or its RNA transcript to produce a potent cell poison is a distinct possibility.
Terminator technology uses a gene coding for an extremely potent cell poison. There is no evidence that the GM crops created can perform as intended. Instead, there is every potential for transgene spread via pollen and horizontal gene transfer, with disastrous consequences for health and biodiversity. The transgenic mustard must not be released.
Article first published 02/09/15
Got something to say about this page? Comment
There are 2 comments on this article so far. Add your comment above.
dhinds Comment left 3rd September 2015 15:03:23
Terminator technology is yet another attempt to privatize evolution for the corporate interest. Congratulations to Mae Wan Ho for informing the public regarding this latest example of deliberate misrepresentation by sociopathic biotech industry criminals, unconcerned and unconscious of the hazards they intend to release on the environment, to the detriment of public health.
Todd Millions Comment left 16th September 2015 23:11:50
'Horizontal'Transfer-Would mutated mustards like Canola be vulnerable too picking up this trait?
So much more than the varieties targeted would be vulnerable too '-pay us or your seed crop won't produce valid product'? How profitable-extortion often is. Just the thing for-'Spawn of wiz drinker Modi' to jump on to.