New Age of water
Entire biochemistry and cell biology textbooks will have to be rewritten on how water in the cell and extracellular matrix is stage-managing the drama of life. This continues the exclusive series started in SiS 23.
Far from being a bag of macromolecules dispersed at random inside a rather tough cell wall, the bacterium is highly and spontaneously organised into nested functional compartments through interactions between the macromolecules and cell water. Dr. Mae-Wan Ho reports
A bacterium is the simplest organism that exists, even though it is by far the oldest, with a direct lineage going right back to the beginning of life on earth some 3.8 billion years ago.
Plants and animals are referred to as eukaryotes, meaning organisms whose cells have a 'true' nucleus, while the bacteria, which have no nucleus, are referred to as prokaryotes. This is indicative of their primitive status as "proto-cells", or forerunners to eukaryotes. But this prejudice is unjustified, say Michael Hoppert and Frank Mayer of Göttingen University in Germany. Frank Mayer, in particular, spent many years studying bacteria, and can vouch for the sophisticated internal architecture that exists in a bacterial cell.
Much of the prejudice against bacteria stems from their small size and the tough cell wall, which make them difficult to study. The much larger plant and animal cells show up many organelles inside such as mitochondria (where food is oxidised to provide energy), lysosomes and peroxisomes (where macromolecules are degraded back into building blocks), and many membrane-bound compartments as well as a cytoskeleton of fibrous proteins that fill the cytoplasm. But a typical electron micrograph of a bacterium on the same scale would reveal an amorphous blob inside.
For a long time, people thought that the bacterium is little more than a rather tough envelope filled with macromolecules scattered randomly throughout the cytoplasm, and that its metabolism is extremely "helter-skelter and inefficient".
In fact, bacteria are stunningly efficient, as is clear from the speed with which they can multiply - doubling every 20 minutes or so in the laboratory - and it makes much more sense to suppose that, even without a membrane, the molecules required for a particular activity are grouped together in what can be called "functional compartments".
The idea of functional compartments is not new, and has been proposed even for eukaryotic cells since the first part of the last century (see The Rainbow and the Worm, the Physics of Organisms). But the evidence for that has so far been indirect.
When bacteria are sufficiently magnified - about one million times - with a powerful enough electron microscopy, an astonishing amount of sub-cellular organisation becomes evident; and it is possible to see several well-defined compartments immediately.
Inside the outer cell wall layers, referred to as the 'capsule', an E. coli cell is further enclosed by two membranes with a space in between - the periplasmic compartment - where nutrients and wastes are captured and sorted, and where a cell-shape controlling network of polysugars and peptides, the peptidoglycan, is located. At the centre of the cell is the densely packed DNA strands of the bacterial genome, folded into a compact body, a nucleoid, forming a loosely defined compartment devoted to storage and use of genetic information. In between the nucleoid and the inner membrane is the cytoplasm, filled with ribosomes (organelles for protein synthesis) and multi-enzyme or multi-protein complexes performing a variety of functions. The most obvious multi-protein complex, connected to the inner membrane, is the flagellar-motor that turns a long, helical flagellum to propel the bacterium through its aqueous environment. Chaperonins and proteosomes are respectively responsible for folding new proteins and disposing of used, obsolete ones. DNA polymerase complexes attached to the DNA strands are responsible for replicating the genetic information. The pyruvate dehydrogenase complex links three sequential reactions together, delivering the metabolites from one reaction to another via a flexible arm of the protein.
But where is the cytoskeleton? Using antibody-staining techniques, Frank Mayer has found evidence of abundant fibrous proteins that form a web-like structure just inside the inner membrane, to which the ribosomes - organelles for synthesizing proteins - are attached.
Thus, there is no doubt that the bacterial cell is just as highly organised as cells of 'higher' organisms.
But how are these functional compartments formed? Studies in the laboratory of Hoppert and Mayer suggest that they form spontaneously as the result of the intrinsic properties of the biological molecules themselves and the way they interact with water in the cytoplasm. Also, the specific structure of water itself could influence the level of enzyme activity in particular microenvironments.
For example, there are compartments called inclusion bodies in certain bacteria, which appear to be storage granules consisting of starch or fats that are not soluble in water. Among the fats often found in inclusion bodies are long chains of fatty acids called polyhydroxyalkanoates (PHAs). Like most fats, they are hydrophobic (water-hating). However, the enzymes that synthesize PHAs are soluble in water. So, while they are synthesized, PHAs are linked to the enzymes and form a complex, part of which is water-soluble and part of which is not. Eventually, the complex rounds up into a spherical compartment in which the water-soluble enzymes form the outer shell, shielding the water insoluble PHAs inside. Water is expelled from the interior, creating an inner compartment separate from the cytoplasm by the water-soluble enzyme molecules. As the PHA inclusion bodies mature, amphiphilic molecules (molecules that love water at one end and hate it at the other) - specific proteins and phospholipids - are added to the growing circumference of the boundary layer, while more PHAs are added to the interior.
Hoppert and Mayer studied enzyme activity in artificial 'reversed micelles'. A conventional micelle is formed when water surrounds amphiphilic molecules that tend to form a sphere, with their water-hating ends inside the sphere and their water-loving ends outside in the water. A reversed micelle is just the opposite. The water-loving ends are inside the sphere interacting with water, while the water-hating ends are outside in touch with a sea of organic solvent.
Depending on the size of the reversed micelle and the location of the water molecules within, the water can adopt two different structures. Water close to the periphery of the micelle in direct contact with the barrier molecules differs from that in the centre, and both are different from bulk water (see Fig. 1). The low-density water forms a lot more intermolecular hydrogen bonds than bulk water, and tends to resemble ice. It also has less charge, is less reactive and more viscous than bulk water. High-density water molecules, by contrast, are less likely to form hydrogen bonds with their neighbours than in bulk water, and are also less free to move about.
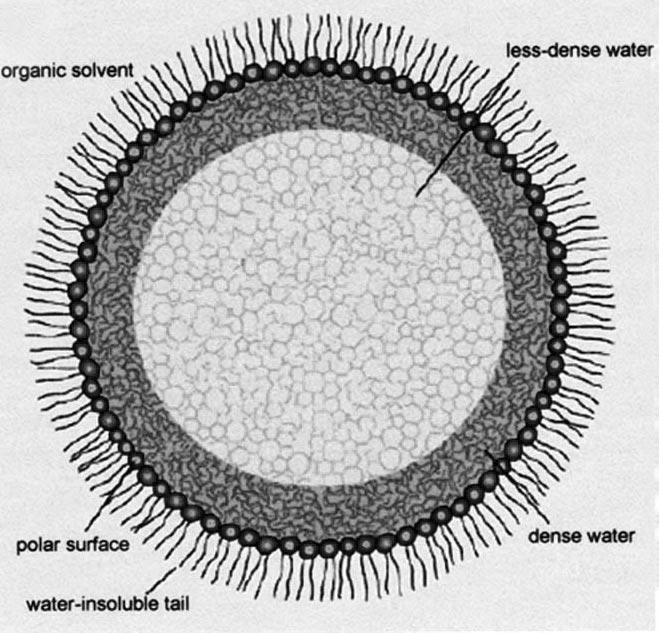
Figure 1. A reversed micelle with enzyme trapped inside.
In the living cell, all compartments and macromolecular assemblies affect water structure, according to Hoppert and Mayer, so there is a non-random variation in high and low density water throughout the cell (see "The importance of cell water", this series), and in turn this would affect the function of the proteins.
It is possible to measure the vibrational frequency of proteins dissolved in low-density water using the reverse micelle system. This revealed that low-density water decreases the vibrational frequency compared with proteins dissolved in bulk water. The vibrational frequency affects enzyme activity. For example, lowering the vibrational frequency of an enzyme may increase the temperature at which the enzyme achieves optimum rate of reaction. Enzymes seem to like low-density water best, where they are presumably more free to move around.
Hoppert and Mayer found that the enzyme activity also depends on the size of the reverse micelles, and remarkably, all enzymes reach optimum activity at a particular size that is approximately the spacing of the periplasmic space in the living cell, about 2 to 10 nanometres wide. Hoppert and Mayer introduced the term ´nanospaces´ to describe them. An organisation into nanospaces is not only found in bacteria; it is also common in any other living cell. This suggests that enzymes may be sitting in a microenvironment of structured water that promotes optimum activity inside the cell. Thus, the layered structure of dense and light water within the cell is part and parcel of the subcellular organisation that enables the cell to function most efficiently.
Article first published 16/10/04
Got something to say about this page? Comment