New Age of water
Entire biochemistry and cell biology textbooks will have to be rewritten on how water in the cell and extracellular matrix is stage-managing the drama of life. This continues the exclusive series started in SiS 23.
It takes life-long commitment, profound knowledge and artistry to show the world what the cell is really like. Dr. Mae-Wan Ho reports
In quest for the secret of life of cells, generations of biologists have dedicated their own lives to finding ways of fixing and freezing tissues so that the structure of cells can be preserved as close to their living state as possible.
But the living 'state' is not a static configuration of structures, but a dynamic process in which structures are constantly changing, constantly being broken down and reformed. And no matter how perfectly preserved, a fixed, frozen section of a cell, like a good photograph of a person, can give no more than an instantaneous snapshot of its life-process.
While we have no difficulty in telling a good snapshot of a person from a bad one - especially if we already know something about the life of the person - there are considerable problems in sorting out actual structures from artefacts of preservation in the case of the cell, especially if we have no idea what the cell is like in real life.
A great deal of aesthetics is involved, both in devising the methods for preservation and in judging which picture best captures the living state. But it is by no means a purely arbitrary aesthetic judgment. On the contrary, it is based on a deep understanding of the living cell and the physics of preservation techniques.
One person who has combined those qualities to an impressive degree is Dr. Ludwig Edelmann in the Saarland University, Homburg, Germany, who has produced some of the most breathtakingly beautiful, 'true-to-life' portraits of cells that I have ever seen. The tenacity and patience with which he pursues his goal is astonishing.
One schedule for preserving rat liver goes as follows: Small pieces of fresh liver were rapidly 'cryofixed' at low, sub-zero temperatures without any chemical fixatives, by placing them on a cold metal mirror. These cryofixed samples were then transferred to a microscope table cooled with liquid nitrogen and cut into thinner slices not thicker than 0.3mm, then transferred into a small metal container (4mm diameter) for a prolonged period of freeze drying at a greatly reduced pressure, so that the ice can sublimate away slowly without disturbing the fine structures of the cells.
The temperature is increased very slowly, at the rate of 0.2C per hour from -90C to -30C, followed by a rate of 1C per hour from -30 to -10C, which took about 13 days, and then maintained at -10C for a further 10 h. In preparation for embedding, the temperature was lowered to -20C and the specimens soaked with components of the resin for 6 hours before warming to room temperature, and the specimens transferred into pure Spurr's resin for 2 to 4 h. Only then were the specimens transferred into embedding moulds containing fresh resin, and allowed to polymerised for 1 day at 60C to give a small solid block out of which ultrathin sections of 60-70nm could be cut with a special diamond knife and stained with uranyl acetate and lead citrate for electron microscopy.
The schedule for rat liver, is not the same as for other tissues. In fact, each cell type or tissue requires a special treatment to give its best results.
Some of the criteria of good results are obvious: high definition of structures, new structures or increased resolution of known structures observed, no shrinkage or swelling, and no breakage of structures. But other criteria are not so clear, and amount to an aesthetic judgement as what is more life-like: a regime in which structures appear as if caught in the midst of casual conversation and trafficking, with each minute entity engaged in its own activity while 'watching' what its neighbours are up to (see Fig. 1). It is a regime of dynamic, spontaneous order in which the structures appear minimally stressed and maximally correlated. It makes you catch your breath in reverence of the beauty of life that has just been unveiled.
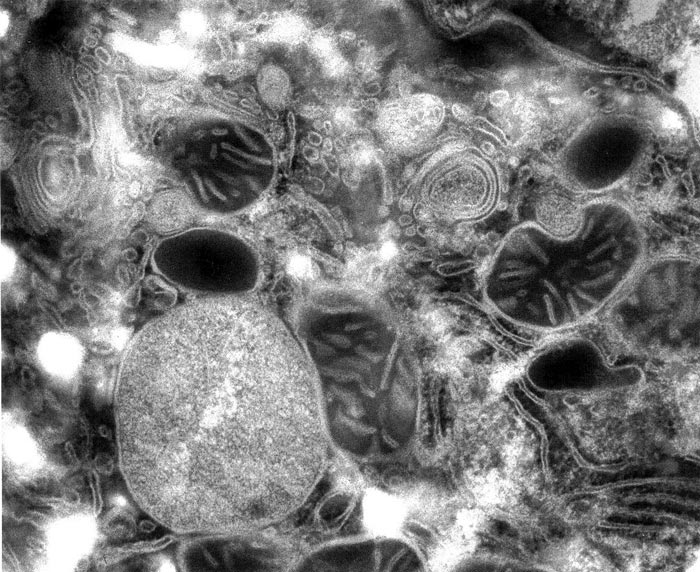
Figure 1. Rat liver cell, magnified 82 000x
Edelman's holy grail for the most life-like picture of the cell goes back a long, long way. Reading Erwin Schrödinger' book, What is Life? convinced him that the living cell is in a state of low entropy, or high degree of dynamic order - an idea that is probably best formulated, he tells me, in the "Association-induction hypothesis" (AIH) that Gilbert Ling proposed in 1962 (see "Strong medicine for cell biology", SiS review). From Ling, he learned that the living cell is primarily an assembly of water, proteins and associated potassium ions, and that the states of water as well as proteins in the living cell are very different from those of bulk water and isolated proteins. Dead cells or cells fixed by chemicals immediately changes this low-entropy (highly ordered) state of water, proteins and potassium ions.
This has spurred him on to find the method that best preserves this living state, and it is slow freeze-drying of cryofixed biological tissues instead of using chemical fixatives and solvents.
In the course of developing these techniques, Edelmann also confirmed a major prediction of AIH, that cellular potassium is adsorbed at negatively charged sites of cellular proteins, and not freely dissolved in cell water as was generally assumed. This assumption inevitably led to the major dogma of contemporary cell biology that Gilbert Ling has thoroughly deconstructed: that a sodium /potassium pump is responsible for pumping sodium ions (Na+) out of the cell and potassium ions (K+) into the cell, thereby keeping intracellular K+ concentration high and Na+ concentration low.
The most spectacular visualization of potassium adsorption was achieved using a method developed by Ling, which was to reversibly replace potassium ions of living muscle cells with chemically similar heavy ions such as caesium or thallium before cyrofixation and freeze-drying. Electron micrographs of thin sections of this muscle demonstrated directly the localisation of the electron-dense heavy metal ions at the myosin protein bands as predicted (see Fig. 2). Edelmann has demonstrated similar localised methods.
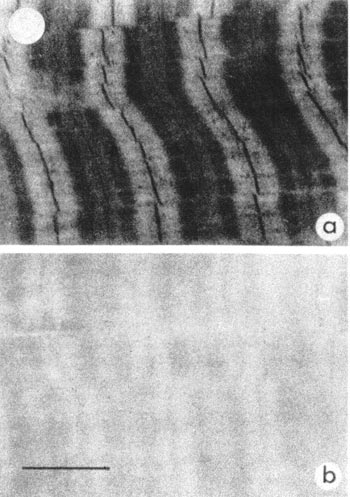
Figure 2. Muscle preloaded with Thallium (a) and containing Potassium (b).
These findings convinced Edelmann that proteins of living cells must have a different structure compared to isolated proteins, which do not selectively adsorb potassium or other similar ions.
In his search of the protein structure in living cells, Edelmann obtained images that have never been seen before. The outer membrane of the cell as well as membranes inside the cell appear in negative contrast, i.e., bright, as opposed to dark, as is usually seen, while proteins of subcellular compartments appear very homogeneously distributed instead of being heterogeneous or fibrous, suggesting that the latter may be artefacts. For comparison with Figure 1, see an area of a liver cell with mitochondria obtained after freeze-substitution involving dehydration at low temperature of a cryofixed specimen with acetone, supplemented with the chemical fixative Osmium tetraoxide (Fig. 3).
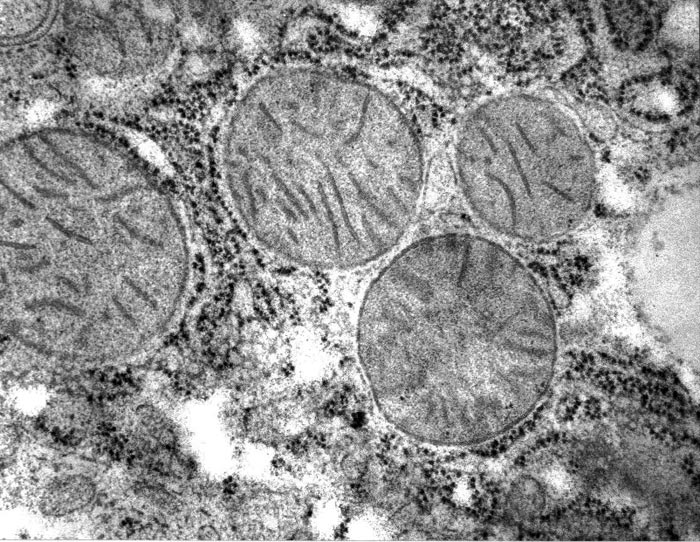
Figure 3 . Rat liver cell freeze-substitution method, compare with Fig. 1.
Edelmann believes that dehydration with organic solvents, as opposed to freeze-drying, both alters the conformation of proteins and removes associated water layers around proteins that are essential for maintaining the original protein structure. He and the world have both been richly rewarded by his sustained efforts.
Article first published 15/10/04
Edelman L. Freeze-dried and resin-embedded biological material is well-suited for ultrastructure research. J Microscopy 2002, 207, 5-26.
Comments are now closed for this article