ISIS mini-series New Age of Water
The age-old alchemist’s dream of turning base materials into gold is coming true in the fabrication of new colloid crystal chips, but the fascinating history of colloid crystals is still unfolding. Dr. Mae-Wan Ho
Colloids are particles of nanometres (10 -9 m) up to several micrometer dimensions (also commonly referred to as nanoparticles) that exist as suspensions in water or other solvents; and colloid crystals are literally crystals made of colloid particles arranged in an orderly way, like atoms in ordinary crystals. Also like ordinary crystals, and macromolecules in living organisms, colloid crystals self-assemble, that is, they form spontaneously when precipitated or evaporated from suspension onto a substrate such as a carbon or silicon-oxide film. The same colloids can self-assemble into a variety of crystals according to the conditions of crystallisation, for example, temperature, pH, ionic and other additives. These three-dimensional colloid crystals are finding applications as electronic and photonic devices [1].
Recently, mixtures of two kinds of colloid particles were found to crystallize together into new and exotic binary nanoparticle superlattice (BMSL) structures. Colloid crystallisation is more an art than science, as the process is still not understood.
In January 2006, researchers at IBM and Columbia University in New York, and University of Michigan Ann Arbor Michigan in the United States succeeded in making more than 15 different BNSL structures using combinations of semi-conducting, metallic and magnetic nanoparticles [2], at least ten of which are new. In many cases, several BNSL structures form simultaneously on the same substrate under identical experimental conditions. The same nanoparticle mixture can also assemble into BNSLs differing in proportion of the two particles and packing arrangement. For example, 11 distinct BNSL structures were prepared from the same batches of 6.2 nm PbSe and 3.0 nm Pd nanoparticles. The structural diversity of BNSLs “defies traditional expectations”, and shows great potential of modular self-assembly at the nanoscale.
The amazing array of colloid crystals now fabricated is the age-old alchemist’s dream come true, base materials are being transformed into an endless variety of exotic electronic and photonic chips worth many times their weight in gold. The history of colloid crystals is no less remarkable, and it is still unfolding.
It started more than 20 years ago when Norio Ise and his colleagues in Osaka, Japan, discovered that solutions of polymers, especially when dilute, are not homogeneous as was previously thought.
Instead, highly ordered and disordered regimes co-exist, as measurements made with small-angle X-ray scattering indicated. In order to see these regimes with their own eyes, Ise and his team used a colloidal dispersion of latex particles 160 nanometre in diameter, which could just be seen under the microscopic; and by means of digital video recording, they were able to demonstrate the two regimes existing side by side (Fig. 1).
The diagram is a composite of a video sequence lasting one second with a frame taken every 1/30 of a second. The average position of the centre of each particle was then computed from the 11th to 20th frames, and the trajectory of the particles during the first ten frames and the final ten frames were plotted from that position.
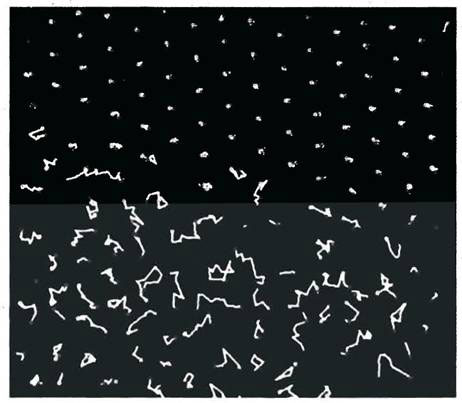
Figure 1. Trajectories of latex particles in the disordered (top) and ordered regime during one second (modified from ref. 3)
As can be seen, the particles hardly moved in the upper half of the field. It was an incredibly ordered regime, against all conventional expectations. The particles at the lower half were in the expected random Brownian motion as described in textbooks. The ordered regime was a molecular super-crystal formed in the water, and these crystals are huge .
Ise and colleagues filled a capillary tube about 50 mm long and 2 mm in diameter with the latex dispersion, and were able to show by means of ultra-small angle X-ray scattering in two dimensions that the entire volume was filled with a single crystal.
In fact, there was a third regime, a void, that contained no particles altogether. This arises naturally in dilute solutions when many ordered regimes or crystals form, and the distance between particles are at their closest about 260 nm (with particles of diameter 112 nm), which is much smaller than the average particle distance. So, when many particles pack together to form crystals, voids will be left behind.
Using a confocal laser-scanning microscope, the researchers were able to follow the formation of void regimes in a dilute dispersion of polystyrene-based latex particles over the course of 60 days (Fig. 2).
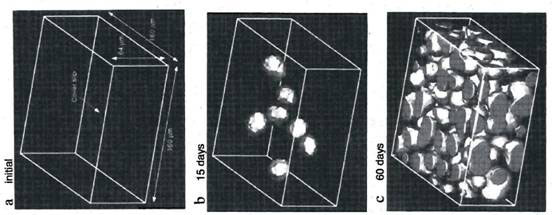
Figure 2. Formation of void regimes in a dispersion of polystyrene latex beads; the voids are represented as bright blobs in the diagram (modified from ref. 3)
Ise and colleagues explained their results by invoking a new long-range attractive force between particles with the same electrical charge. This was really unheard of, not just in colloid science, but in the foundations of chemistry. Like charges repel, only opposite charges attract. The conventional wisdom in colloid science, due to Derjaguin, Landau, Verwey and Overbeek (the DLVO theory for short), is that a few static charges on the colloid particles’ surfaces can cause repulsion strong enough to keep them stably separated, and that’s why the particles stay dispersed. Particles with the same charge (negative charges in the case of the latex particles) can only repel one another at short range (nanometres); otherwise, they are shielded from each other’s repulsive influences by ‘counter-ions’ (ions of opposite charge from those on the colloids), so the interaction drops off exponentially with distance and nothing in the counter-ions can mediate attraction.
But according to the experiments of Ise and colleagues, there was indeed an attractive force between like charges at distances of 5-50 microns, which could be demonstrated also between the negatively charged latex particles and the negatively charged glass wall that contained the latex dispersion. And the more highly charged the particles, the stronger the attraction.
At first the results from Ise’s laboratory were treated with utter scepticism by the scientific establishment, and attributed to artefacts such as unclean glassware. But other laboratories have repeated the results since, though the explanation remains illusive.
David Grier and colleagues at the University of Chicago Illinois in the United States, used optical tweezers (laser light) to position two polystyrene beads of radius 482 nm in deionised water, and measured the interaction between the pair by tracking their motions with digital video microscopy [4]. The interaction potential between the pair of charged colloidal spheres was purely repulsive, as can be seen in Fig. 3a. But when the same pair of spheres was confined between parallel glass walls separated by 3.5 microns, an attractive (negative energy) minimum develops in the interaction potential at a separation distance of about 2 microns (Fig. 3b). Attractions of about the same range and strength were thought to be involved in the formation of superheated colloidal crystals (c).
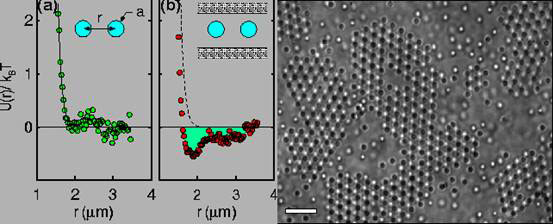
Figure 3. Interaction potential between two polystyrene spheres in free solution (a) and confined between glass plates (b). Superheated colloid crystals showing disordered and void regimes, scale bar 20 microns (c) (modified from ref. 4)
Doubt arose again later when the attractive force was only found in particles narrowly confined between glass walls that were thought to be largely responsible for the attractive forces [5]. Explanations proposed to account for the effect [5, 6] include a strong non-linear coupling between the colloid ions and the simple counter-ions shielding them from one another, so that electrical neutrality is no longer satisfied, and a residual electrostatic attraction develops between the particles.
One aspect largely ignored is the role of water. Work done by Kinoshita and colleagues at Kyoto University, Japan, showed that including an ionic, dipolar solvent such as water results in an effective attraction between the colloid particles, and when the size of the counter-ions is sufficiently large, and the ionic concentration sufficiently high, the interaction between highly like-charged colloid particles can be strongly attractive [7].
But simple attraction could simply result in the colloid particles aggregating and precipitating out. It still does not explain why the particles should form these extraordinary large crystals in the water. Perhaps the key lies in the structure of water itself. Could it be that the charged particles in the colloid crystals of the ordered domains discovered by Norio Ise and coworkers (Fig. 1) are sitting among and stabilising structured water similar to the expanded icosahedrons proposed by Martin Chaplin? Read Two-states Water Explains All? (this series).
The amazing organising properties of water are becoming more and more evident, which will go a long way towards explaining the detailed organisation of molecules in cells and their biological functions (Water and Effortless Action at a Distance, this series).
Article first published 26/10/06
Comments are now closed for this article