ISIS mini-series New Age of Water
Water plays the lead role in living processes through changing between two states, a brand new understanding of bioenergetics and enzyme action. Dr. Mae-Wan Ho
Bioenergetics is the big problem of how living organisms capture and transform energy for growth, development, and all that life entails, and it fills many pages in standard biochemistry textbooks.
In a nutshell, green plants capture energy from the sun in photosynthesis to make simple carbohydrates (the building blocks for everything else) from carbon dioxide and water. In respiration, carbohydrates are broken down ultimately back into carbon dioxide and water, and along the way, energy is abstracted to make ATP. ATP is also made in photosynthesis, but the most important job of photosynthesis is capturing carbon.
ATP (adenosine triphosphate)
is the ‘high energy intermediate’ or ‘energy currency’ that fuels all living
processes: synthesizing proteins, DNA, RNA, carbohydrates and fats, operating
molecular motors, ion pumps, and so on. The free energy of hydrolysis of ATP
is transferred to the enzyme involved, which then uses the energy to perform
the work of transport, or sliding filaments, or synthesis of
peptides, olignucleotides etc.
But that’s a myth, says Philippa Wiggins of Genesis Research and Development Corporation in Auckland, New Zealand, who has spent nearly 30 years investigating the role of water in living processes [1].
Wiggins does not question the observation that ATP hydrolysis - (reaction with water) into ADP (adenosine diphosphate) and Pi (inorganic phosphate) - is an essential part of many reactions in living organisms. She questions the central idea that ATP hydrolysis is necessary and sufficient for transforming energy, while the role of water as solvent and organising medium for the reactions is completely ignored.
Many of the chemical reactions taking place in living organisms require extraordinarily harsh conditions if done in a test tube without enzymes. So the story goes that enzymes can work miracles by “conformational” (shape) changes, again ignoring the role of water.
For example, to break peptide bonds in proteins or nucleotide bonds in DNA or RNA would required boiling in strong (6M) hydrochloric acid, whereas with the enzymes, protease, DNAse and RNAse, the bonds are readily broken at neutral pH and ambient temperature. Similarly, our bodies make bones (consisting largely of calcium phosphate Ca 3 (PO4 )2 ) continuously (and break them down continuously too) from very dilute solutions of calcium and phosphate at pH 7.4 and 38 C. The salts crystallise out from water in conjunction with specialised bone-forming cells, the osteoblasts. To make similar ceramics requires a temperature of 1 200 C. It is more likely the change in physical chemical properties of water that enable enzymes to work and salts to crystallise out from dilute solution.
Wiggins is not alone in thinking that water is the lead player in living processes. Gilbert Ling [2] has been criticising the conventional account of energy transformation since the 1950s and proposed a comprehensive alternative theory based on water organised in extended dipolar layers on surfaces of membranes and proteins, which power biochemical reactions through abrupt phase changes on interacting with ATP and inorganic ions [3] (Strong Medicine for Cell Biology). The theme of phase transitions in biological interfacial water was taken up by muscle biochemist Gerald Pollack in his book, Cells Gels and the Engines of Life [4], which I have reviewed as Biology of Least Action [5].
Wiggins subscribes to the two-states model, which proposes that liquid water at ambient temperatures is a mixture of domains of low-density water (LDW) with intermolecular bonding similar to ice 1h and domains of high-density water (HDW) with intermolecular bonding similar to ice II (Two-States Water Explains All? this series). This model explains many seemingly baffling and contradictory observations that she and her colleagues and other researchers have made over the years, and more importantly, tell a much more coherent story of how water, by changing its physical and chemical properties, can make apparently impossible chemical reactions happen effortlessly in living organisms.
Wiggins and colleagues began experimenting in the 1980s with cellulose acetate films that have small pores of about 2 nanometres in diameter. By soaking the films in aqueous solutions containing different salts, they discovered how different ions completely changed the physical and chemical properties of water.
They found that the water in the pores of films soaked in water, NaCl, LiCl or MgCl 2 solutions had the infrared spectrum of ice 1h, like LDW, whereas films soaked in solutions of KCl or CsCl had the spectrum of liquid water.
The water in the pores was also highly selective to ions. KCl and CsCl were accumulated if the external concentrations were small, and as the external concentration increased, then the concentration in the pores became equal to the external. NaCl, LiCl, HCl, CaCl 2 and MgCl2 were increasingly excluded from the pores; and their degree of exclusion increased with the external concentration. The selectivity to K+ versus Na+ is similar to that of the cell, which has high concentrations of K+ inside and low concentrations of Na+ , the reverse of the situation in blood and extracellular fluids. This selectivity has prompted Ling [3] and others [6] (What's the Cell Really Like?) to conclude that the cell, in common with other polyelectrolytes (materials like cellulose acetate with many electrical charges), does not need its membrane to keep Na+ out, nor Na+ pumps to actively pump it out of the cell by consuming ATP.
Wiggins and colleagues concluded that KCl and CsCl were selectively accumulated into the ice-like LDW in the pore, but that at high concentrations, made LDW revert to normal water. NaCl, LiCl and MgCl 2 , on the other hand, were selectively excluded, and imposed an osmotic pressure gradient on the pore water, causing it to decrease in density and increasing the exclusion of the ions.
What’s the evidence that an osmotic gradient due to excluded ions is involved? Adding an excluded salt such as MgCl 2 to the external solution to balance out the osmotic pressure gradient increases the accumulation of KCL in the pore water.
After many experiments of a similar nature, not only with cellulose acetate films, but also with microporous polyamide beads and hydrophobic glass beads, and investigations on the changes in viscosity of water with different solutes, Wiggins proposed a dynamic theory of phase changes in water induced by ions and other small molecules.
The two states model of water is represented as domains of HDW mixed with LDW, as opposed to the single-state homogenous water (see Fig. 1).
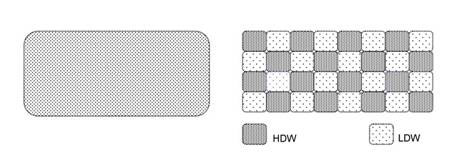
Figure 1. Diagrammatic representation of single-state homogeneous water (left) and two-states water with mixed domains of HDW and LDW (modified from ref.1)
A solute in single-state water will experience a single environment, whereas in two-states water, the solute will partition differently in the two kinds of domains according to its preference for the different domains. Consequently, instantaneous gradients will be set up between neighbouring unlike domains. In Figure 2, the solute has preference for LDW; this sets up local gradients in osmotic pressure, which is eliminated by the transformation of LDW into HDW.
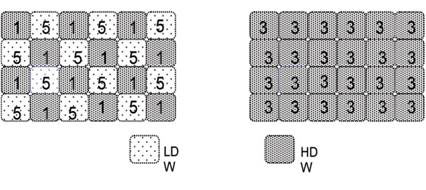
Figure 2. A solute that partitions preferentially into LDW domains (left) creates local gradients in osmotic pressure that are eliminated by conversion of LDW domains into HDW domains (modified from ref.1)
Conversely, a solute that partitions preferentially in HDW will create local gradients that are eliminated by conversion of HDW into LDW.
In other words, there are two classes of solutes, those that prefer LDW and others that prefer HDW, and once they partition into their preferred domain, they tend to transform it into the other due to the local osmotic gradient created. And that’s how a dynamic cycle can be kept going. It is really quite ingenious, and can explain many of the miraculous things that enzymes and ATP are supposed to do.
The two classes of solutes also happen to correspond to the traditional distinction between chaotropes (K + , NH4 + , Cl- , Br- , HCO3 - , HSO4 - , H2 PO4 - ) that decrease the structure of water (by inducing HDW), and kosmotropes (H+ , Li+ , Na+ , Ca2+ , Mg2+ , hydrophobic non-electrolytes) that increase the structure of water (by inducing LDW, which has lower entropy than HDW).
One of the most convincing evidence for water’s effortless action – which requires no energy input – is the synthesis of ATP from ADP in the LDW within the pores of cellulose acetate films. In the experiment, ADP was converted to ATP in the presence of nothing but potassium phosphate (K 3 PO4 ) to provide inorganic phosphate. It was a spontaneous reaction in LDW, and the K+ -induced transformation of the LDW to HDW released the ATP (and associated K+ to the external solution) so the dynamic cycle can be repeated. When the external solution contained NaCl or MgCl2 , however, no ATP was detected. That was because the ATP formed in LDW in the pores are trapped inside, and cannot be released because NaCl and MgCl2 stabilises LDW, and the dynamic cycle was interrupted.
The cell makes ATP by the membrane-bound enzyme ATP synthase, requiring a flux of H + through the enzyme, and down a concentration gradient. The concentration gradient across the membrane was built up by abstracting the energy from food (in respiration) or from the sun (in photosynthesis). (The existence of a concentration gradient in the bulk phase is hotly disputed, however. Many scientists now believe that H + ions simply migrate along one side of the membrane to the ATP synthase.) According to Wiggins, the flux of H + ions is required, not to provide energy for ATP synthesis, but to break down the LDW formed within the cavity containing the active site of the enzyme, allowing the release of spontaneously synthesized ATP.
How does the hydrolysis of ATP, proteins and nucleic acids, etc., by enzymes take place?
Water is a very weak acid and base. The concentrations of H + and OH- ions are each at 10-7 M at 25 C. This again is unusual because oxygen containing acids of neighbouring elements in the Periodic Table are often very strong, such as H2 SO4 and H3 PO4 . The reason is that H+ is the most powerful kosmotrope of all univalent cations, and the OH- ion is one of the very few kosmotropes among univalent anions (negatively charged ions). Water ionises in HDW, but not in LDW; but the immediate consequence of ionising in HDW is to convert the HDW to LDW to eliminate the steep osmotic pressure gradient created between neighbouring domains, so liquid water is largely unionised.
Strong acids such as H 3 PO4 ionises first to produce the powerful kosmotrope H+ and the powerful chaotrope H2 PO4 - , so there is practically no osmotic imbalance created, and hence little conversion of LDW into HDW domains or vice versa . In this case, water remains ionised in the HDW domains.
In order to carry out hydrolytic reactions, there must be sufficient ionised H + and OH- ions present.
This suggests how enzymes that carry out hydrolytic reactions might work. Proteases, for example, often require Ca 2 + , which induces HDW, thereby protecting the ionisation of water, so water surrounding the peptide bond can act as strong acid and strong base to break it. The same would apply in the case of DNAse and RNAse that break polynucleotide bonds.
Similarly, in the operation of the sodium pump, the Na, K-ATPase, which ‘pumps’ Na + out of the cell in exchange for K + , it is the change in water structure that provides the decisive, effortless action, rather than the ‘free energy’ from the hydrolysis of ATP into ADP.
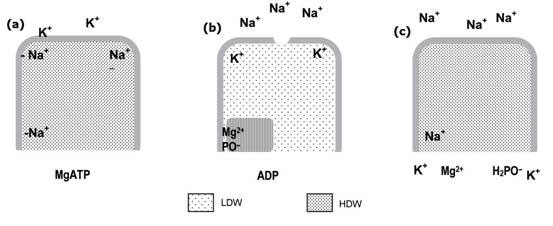
Figure 3. How the Na K-ATPase enzyme works (modified from ref. 1, see main text)
Figure 3 shows how the enzyme could work: (a) Na,K-ATPase cavity with the active site of the enzyme in its resting state contains HDW with Na+ as counter ion to the aspartic acid residue that’s to be phosphorylated, and to two negative sites near the top of the cavity; (b) MgATP phosphorylates the aspartic acid residue, Mg 2 + remaining as counter ion, LDW is induced and moves up the cavity, pushing Na + out ahead of it through the open channel as K enters selectively into the LDW; (c) the K + ions induces HDW to balance the osmotic gradient created and ionises water, so the phosphate is hydrolysed off; K + , Mg 2 + , phosphate and ADP diffuse out and Na + re-enters to neutralise the aspartic acid residue. Two more ions enter in exchange for K + and the cavity reverts to its resting state (a).
I don’t think we have all the answers yet to the strangeness of water; but I do get the feeling that water is both concertmistress and lead player in the Quantum Jazz of life (this issue).
Article first published 27/10/06
Got something to say about this page? Comment