New research reveals disastrous ecological impacts of the world’s top herbicide and GM crops made tolerant to it Dr. Mae-Wan Ho and Brett Cherry
Glyphosate tolerant (GT) crops and glyphosate herbicide (commercial formulation, Roundup) poison nitrogen fixing and other beneficial soil bacteria, increase fungal pathogens, undermine plant immunity to diseases, decrease plant micronutrients available in the soil, and more.
Research findings over the past decades paint a damning picture of the cropping system that has taken over 85 percent of the 134 million hectares of global agricultural land now growing genetically modified (GM) crops (see [1] Scientists Reveal Glyphosate Poisons Crops and Soil, SiS 47). The unprecedented rise in GT crops has been accompanied by a sharp increase in the use of the glyphosate herbicides worldwide, especially in the US [2] GM Crops Increase Herbicide Use in the United States, SiS 45).
The ecological disaster has been unfolding amid mounting evidence of the herbicide’s adverse impacts on human and animal health [3, 4] (Glyphosate Herbicide Could Cause Birth Defects, Ban Glyphosate Herbicides Now, SiS 43), and the breakdown of the Roundup Ready (RR) cropping system as weeds and superweeds become resistant to the herbicide [5, 6] (GM Crops Facing Meltdown in the USA, Glyphosate Resistance in Weeds - The Transgenic Treadmill, SiS 46) [7].
Glyphosate (N-(phoshonomethyl)glycine) (Figure 1) is a broad-spectrum herbicide initially patented by Monsanto in the 1970s under the trade name Roundup.
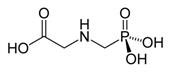
Figure 1 Glyphosate (N-(phoshonomethyl)glycine)
Glyphosate kills plants by binding to and inhibiting the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) of the shikimate pathway for the synthesis of the aromatic amino acids, phenylalanine, tyrosine and tryptophan. These amino acids are essential building blocks for all proteins, and also precursors for growth factors and phytoalexins, compounds involved in the plant’s defence against diseases [8, 9]. Animals do not have the shikimate pathway and depend on getting the essential amino acids from their diet.
GT plants depend on incorporating an EPSPS from the soil bacterium Agrobacterium tumefaciens (which causes crown gall disease) that is insensitive to glyphosate, and hence not killed by the herbicide.
For a long time, glyphosate has been promoted as the safest and most environmentally benign herbicide available. But glyphosate has many other effects that act synergistically on crop health and productivity that extends well beyond the plant into the soil ecosystem and the wider environment.
The first hints of these effects came from observations that glyphosate application greatly increases the severity and incidence of plant diseases, not just in the GT crops, but also in subsequent crops grown in the same soil.
Glyphosate increases plant disease by several mechanisms that weaken the plant and its defences against disease, and at the same time boosts the virulence of pathogens and their populations in the soil. What makes glyphosate such a strong herbicide is that it is translocated throughout the plant to the growing points of shoots and roots to make the susceptible plants stop growing. In the roots, glyphosate is exuded into the rhizosphere (the soil surrounding the roots) where it exerts powerful effects on the microbial community and soil chemistry.
Chemically, glyphosate is a strong chelator (binder) of metal ions, rendering these essential nutrients unavailable in the soil and within the plant. Glyphosate was patented as a strong chelator in 1964. In contrast to many chelators that bind specific metal ions, glyphosate is a broad spectrum chelator that binds both macro and micronutrients such as Ca, Mg, Cu, Fe, Mn, Ni and Zn. It is that which makes it a broad spectrum herbicide as well as a potent antimicrobial agent, as the function of numerous enzymes depends on specific metal co-factors [10].
The EPSPS enzyme, for example, requires manganese (Mn) as co-factor, as does 25 other plant enzymes, and glyphosate reduces the availability of Mn for all of them. In addition, because the herbicide also chelates other metal ions, it interferes with a wide range of biological functions, thereby weakening the plants, making them more susceptible to diseases, and reducing productivity. This applies to GT as well as non-GT plants. Thus, GT plants are weakened, though not killed by the chelating action of glyphosate, which accounts at least in part for the reduced yield of GT crops.
As glyphosate is exuded through the roots of GT crops or dying weeds that have been sprayed, the herbicide is rapidly taken up by other plants or immobilized in the soil by binding to metal ions, so that the ions are no longer available for uptake by the plants. Glyphosate can remain in the soil for a very long time, which is just as well, because its degradation products are toxic to GT plants and non-GT plants. But that means crops subsequently grown on the same soil will still be exposed to high levels of the herbicide, and the effects accumulate as more herbicide is sprayed.
Glyphosate reduces nitrogen fixation by several mechanisms. Nitrogen-fixing bacteria such as the soybean symbiont, Bradyrhizobioum japonicum, possess a glypohsate-sensitive EPSPS, and hence fail to grow when exposed to glyphosate. This may be another significant factor in the reduced growth and yield of GT soybean.
N-fixation is also affected indirectly via the physiology of the host plant. Glyphosate inhibits the formation of the growth factor Indoylacetic acid IAA in GT soybeans, which leads to lower root nodule formation by the symbiont. Glyphosate forms several metabolites such as aminomethlphosphonic acid (AMPA), sarcosine and glycine. Chlorotic symptoms of GT soybean following glyhosate application have been attributed to the accumulation of AMPA.
Nickel (Ni) is involved in N fixation via the Ni-requiring hydrogenase activity that recycles hydrogen to provide energy for nitrogen fixation. So lack of Ni in soils due to glyphosate chelation can limit the sympbiotic bacteria’s hydrogenase activity. When hydrogenase is inhibited, some 30 to 50 percent of the energy supplied to nitrogenase can be lost as H2, greatly diminishing the efficiency of N fixation
Tests were carried out at the State University of Maringa, Parana, Brazil, in both a clayey and sandy soil with seeds of near-isogenic normal and GT soybean varieties treated with fungicide [11]. The tests showed that glyphosate reduced N fixation independent of soil type and cultivar.
Shoot and root dry weights were both reduced by glyphosate application. AMPA damage to chlorophyll could reduce shoot growth. Glyphosate treated plants exhibited chlorotic symptoms (yellowing) compared to plants without glyphosate, reflecting damage to chlorophyll and decreased photosynthetic rate.
Chlorophyll content in leaf was indeed lower, probably due to direct damage by AMPA or the chelation of Mg components of chlorophyll or Mn involved in electron transfer during photosynthesis.
Herbicides are known to increase specific plant diseases since the 1970s.
Glyphosate inhibits EPSPS both directly and via Mn chelation (see above). Plants with a compromised shikimate metabolism are predisposed to various plant pathogens, and glyphosate is actually patented as a synergist for mycoherbicides to enhance the virulence and pathogencitiy of fungi used for biological weed control. The synergistic activity of glyphosate weed control in predisposing plants to infectious organisms has been observed for many diseases and the extensive use of glyphosate in agriculture is a significant factor in the increased severity or “re-emergence” of diseases once considered efficiently managed [12].
The toxic effects of glyphosate are cumulative, and become worse with continued use, so Mn deficiency is now observed in areas that were previously Mn sufficient, partly because of the accumulation of glyphosate itself in the soil and also because glyphosate poisons and depletes the populations of Mn-reducing soil organisms. Reduced Mn is taken up by plants, whereas oxidized Mn is not; hence the balance of Mn-reducing microorganisms in the soil is crucial to Mn availability to plants. The presence of glyphosate-tolerance gene also reduces Mn uptake and physiological efficiency (through the accumulation of glyphosate in the plant), and concomitantly, increases disease severity.
The virulence of some pathogens such as the fungi Gaeumannomyces, Magnaporthe, Phymatotrichum and Corynespora, and the bacterium Streptomyces, involves Mn oxidation at the site of infection (Mn2+ to Mn4+), which compromises the plant’s resistance via the shikimate pathway; as oxidized Mn is of no use for the enzymes in the pathway that uses Mn as co-factor.
Some 40 diseases are known to be increased in weed control programmes with glyphosate and the list is growing [1, 12], affecting a wide range of species: apples, bananas, barley, bean, canola, citrus, cotton, grape, melon, soybean, sugar beet, sugarcane, tomato and wheat.
Corynespora root rot of soybean, previously considered minor, may become economically damaging in RR soybean. This fungal root rot is more severe when glyphosate is applied to soybeans under weedy conditions even though the weed may not be hosts for the fungal pathogen. The reason is that the weeds serve to translocate and release more glyphosate into the rhizosphere, to deplete the populations of Mn-reducing organisms, and diminish manganese availability for plants to shore up their defence. All that acts synergistically to boost the increase of Corynspora and its ability to cause disease.
“Take-all” disease of cereal crops increases after a pre-planting “burn-down” of weeds with glyphosate; and this has been recognized for more than 15 years. The disease is also increased when glyphosate is applied to RR soybeans the preceding year compared with the use of a non-glyphosate herbicide. Again, this is due to reduced availability of Mn; all factors that reduce Mn availability, such as low soil pH, overuse of N fertilizers, also increase the severity of the disease. Microorganisms proposed for biological control of the disease such as the bacterium Bacillus cereus and fungus Trichoderma konigii are all strong Mn reducers that increase Mn availability in the rhizosphere. In contrast, addition of Mn oxidisers increases take-all.
Thus, in addition to its chelating action that makes Mn unavailable, glyphosate poisons Mn-reducing and N-fixing organisms in the soil, so the availability of both N and Mn for crop plants could be markedly compromised.
Increased take-all root, crown, and foot rot of cereals following application may result from the synergistic effects of reduced resistence due to Mn deficiency, inhibition of root growth from glyphosate accumulation in root tip, and increase in Mn-oxidizing organisms and diminished Mn reducing organisms in the soil community.
Diseases caused by the fungus Fusarium have increased with the extensive use of glyphosate [12]. For example, glyphosate use predisposes tomatoes to Fusarium crown and root rot. Cotton growers in Australia and the Western USA have seen a resurgence of Fusarium wilt since the introduction of Roundup Ready cotton, and previously high levels of wilt resistance appear to be less effective under glyphosate management Fusarium head scab of cereals and other Fusarium diseases increase follow glyphosate applications. Head scab and the mycotoxins produced by the causal fungi are now prevalent in cooler areas where they were rare before the extensive use of glyphosate.
The Palouse area of Washington, Idaho, and Oregon in the US has had a long history of pea, lentil, and wheat production on the deep loess soils characteristic of the area. However, pea and lentil yields have been in slow decline as symbiotic nitrogen fixation is reduced and Fusarium diseases increased with the extensive use of glyphosate for no-till wheat production. Pea and lentil production are now uneconomical in some farms, and production is rapidly moving from the Palouse to Montana where glyphosate use has been more limited.
A new Fusarium wilt of canola caused by F. oxysporum and F. avenaceum has severely reduced yields in the nutrient poor soils of Alberta and Saskatchewan in Canada since 2000.
Sudden death syndrome (SDS) of soybean reached “epidemic proportions” in North and South America in the late 1990s [13]. It remains widespread in the soybean-growing regions in the US, Argentina and Brazil, and is caused by two distinct species: F. viruliforme in North America and F. tucumaniae in South America. The increased use of glyphosate was identified with SDS, especially in years of increased rainfall [14].
Research dating back to the early 1980s revealed that the herbicidal efficacy of glyphosate is largely due to the colonization of roots of affected plants by soil-borne pathogens (rather than the inhibition of aromatic amino acid synthesis as originally thought). The two most important pathogens in this regard were the fungi Pythium and Fusarium, both ubiquitous in soils.
Robert Kremer, a microbiologist with the USDA, and his research team has conducted studies in Missouri from 1997 to 2007 to assess the effects of glyphosate on GT soybean and GT maize root colonisation, and soil populations of the fungus Fusarium along with other rhizosphere bacteria [15]. They found that the roots of GT maize and soybeans treated with glyphosate were heavily colonised by Fusarium compared to GT and conventional crops not treated with glyphosate. Their findings suggest that glyphosate exuded by the plant roots may even serve as a nutrient source for fungi and stimulate the germination of Fusarium spores, as Fusarium dominated the fungal community of the rhizosphere after long term exposure to glyphosate.
A significant increase in Fusarium was detected in just two weeks after the application of glyphosate at recommended doses, and was two to five times higher in GT soybean treated with glyphosate than non-treated GT or conventional soybean (Figure 1, top). GT maize treated with glyphosate had Fusarium colonisation three to ten times higher than when the herbicide atrazine was used instead (Fig. 2, bottom).
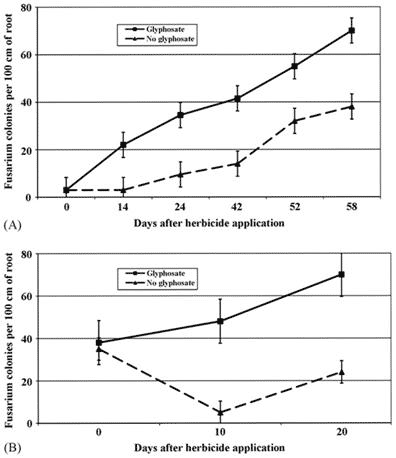
Figure 2 Fusarium colonisation of glyphosate-resistant (GR) crops with and without glyphosate applications [9]
Another study based on crop field surveys and large-scale experiments in Saskatchewan, Canada, singled out glyphosate application as the most important factor in the development of crop diseases. Head blight in barley and wheat crops caused by Fusarium graminearum was especially widespread. Fusarium graminearum was the most common pathogen isolated in the four year study, infecting 41.3 percent of the common and durum wheat analysed. According to the study [16], “...growing susceptible crops under minimum-till management in fields where glyphosate has been previously applied, resulted in the most damage from FHB (Fusarium Head Blight) in years conducive to disease development.” This is especially worrisome as Roundup crops were designed specifically for farmers to avoid tilling and due to this “convenience” they may actually be inadvertently preparing their crops for head blight.
Kremer personally went to USDA administrators urging the agency to publish a news release on the numerous studies confirming the environmental impacts of GT crops and glyphosate, but was ignored. “Their thinking is that if farmers are using this (Roundup Ready) technology, USDA doesn’t want negative information being released about it,” he said [17].
Research published in 1979 already showed that glyphosate absorbed through plant foliage after application was transported systemically toward the roots and eventually released into the rhizopshere [15] where it changes the whole ecology of the soil, resulting in increased colonisation of plant roots by pathogenic species such as Fusarium and Phytophorthora, as well as Pythium in bean plants.
In addition, glyphosate increases excretion of substrates from roots that may be selectively metabolized by pathogens, such as amino acids and glyphosate itself, thus encouraging these pathogens to flourish. In the meantime, glyphosate and its breakdown products such as AMPA are poisonous to susceptible organisms, many of which are beneficial.
Pseudomonas are important multifunctional bacteria in the rhizospere that produce numerous secondary metabolites that suppress harmful microbes such as fungal pathogens, including Fusarium, and contribute to reducing Mn, thus making it available to the plants. Glyphosate and GT soybean significantly decrease these beneficial Pseudomonas species in the rhizosphere, thereby further encouraging the growth of the fungal pathogens by suppressing their bacterial antagonists.
Mn oxidation and reduction are primarily carried out by bacteria in the rhizosphere, and have a major impact on plant nutrient availability and metabolism. A low ratio of Mn reducers to Mn oxidizers was found for GT soybean treated with glyphosate compared with non- GT soybean, suggesting less Mn is available for the plant. In addition to suppressing Mn reducing Pseudomonas, glyphosate enhances Mn oxidizing bacteria, most likely Agrobacteria that typically form biofilms on the soybean root surface. The oxidized Mn are retained within the biofilm. These Agrobacteria probably have glyphosate-insensitive EPSPS, similar to the Agrobacterium tumefaciens that supplied the enzyme for GT soybeans.
As mentioned earlier, glyphosate also inhibits the growth of the N-fixing symbiont of soybean.
Glyphosate released through the roots of dying plants (weeds) is transferred to living plants not treated with glyphosate via the roots, suggesting that glyphosate applied to weeds or other vegetation in orchard alleys may be similarly transferred to trees causing disease and yield losses [15]. There is evidence that such transfer to other plants via the roots of dying plants is much more effective than direct spraying into the soil.
A green house experiment showed that Roundup Ultramax (from Monsanto) sprayed on ryegrass at killing doses was much more effective in inhibiting the growth of sunflower seedlings sown in the same soil than the same amount of herbicide mixed directly into the soil [18]. The growth inhibition was most pronounced when there was no lag time between the application of the herbicide and the sowing of the sunflower seeds. The growth inhibition effect wore off between 7 to 21 days after herbicide application, though it did not disappear entirely. With no lag time before sowing the sunflower seeds, growth inhibition was 90 percent when herbicide was applied to ryegrass, compared to 50-70 percent when herbicide was mixed into the soil.
The increase in shikimate with glyphosate application through ryegrass weed was 10 to 100 fold that of direct application to soil. Glyphosate poisoning of sunflower seedling was associated with an impairment of the manganese-nutritional status, which was still detectable after a waiting time of up to 21days (see Figure 3).

Figure 3 Effect of glyphosate application through weed compared with application directly to soil [18]
In most plant species, glyphosate is not readily metabolized and is preferentially translocated to young growing tissues of roots and shoots, where it can accumulate at substantial concentrations. This can easily create hotspots in the soil containing high levels of glyphosate. The findings of the study are in line with field observations of plant damage in winter wheat in no-till systems after glyphosate applications before sowing and lag times shorter than two weeks.
It is clear that GT crops and accompanying glyphosate use are serious threats to the sustainability of agriculture and food production. These extensive ecological impacts of glyphosate and the Roundup Ready cropping system should be seen in the light of accompanying threats to human and animal health that already justifies a global ban on the use of the herbicide [40]. It is indeed time to stop both glyphosate and glyphosate GT crops, and indeed all GM crops and concentrate resources on localised organic agricultural systems that have proven to work sustainably and more productively that chemical industrial agriculture (see [19] Food Futures Now: *Organic *Sustainable *Fossil Fuel Free).
Article first published 26/05/10
Comments are now closed for this article
There are 13 comments on this article.
Dean Mindopck Comment left 27th May 2010 20:08:46
work sustainably and more productively that chemical industrial agriculture (see [19] Food Futures Now: *Organic *Sustainable *Fossil Fuel Free).
Typo in above fragment. Suggest replace "that" with "than".
Peter Brenton Comment left 30th May 2010 20:08:49
Now lets get everyone concerned about the use of Round-up and the fact it is produced by the organic world's biggest threat Monsanto , to campaign strongly against its presence in foodstores and garden centres
Ed flugel Comment left 26th November 2010 19:07:01
I wish I knew about this in the spring, yes I have a well. I will need to change weed practices.
Mae-Wan Ho Comment left 9th June 2010 16:04:49
John wilson, yes, glyphosate can become unbound when the soil is rich in phosphate, as when manure or artificial fertilizer has been added, then glyphosate leaches from the soil to contaminate ground/drinking water.
John Wilson Comment left 9th June 2010 16:04:23
Many thanks for this extremely thorough and hard hitting article. One query.
I have read that glyphosate can become unbound from soil particles. i.e. It may not be permanently immobilised by metal ions in the soil. Could the glyphosate then enter soil water and eventually migrate into streams and rivers ?
Ai Wai Comment left 19th August 2011 17:05:19
Oh dear... I should have read this a year ago. I sprayed my durian orchard with Roundup to get rid of 'lalang' (Imperata cylindrica) a noxious weed that spreads through underground rhizomes. Then I observed defoliation, death of branches, and phytophtora infestation on some trees. The problem is there don't seem to be other weedkillers than can effectively eradicate the particular weed - slashing makes it even worse.
Abdur Rashid Comment left 2nd December 2011 05:05:56
I am presently doing some experiments and I require time to comment here.
Nattalia Comment left 30th May 2013 16:04:15
Question: What happens to marine plants if there is run-off of
glyphosates into water bodies?
David R Comment left 31st August 2014 02:02:48
Is there a connection between glyphosate and uneven ripening in bananas?
Mr.gituanja Gachie Comment left 22nd October 2014 16:04:48
It is high time we all rise against this corporate greed, and let polluter pays principle apply.They got away with DDT and now Persistent organic pollutants.We as consumers have a choice of boycotting food with gm ingredients that way we can fight corporates.
jan Comment left 18th March 2015 01:01:17
Very good article, very thorough explanation
Dianne Cobb Comment left 8th September 2015 16:04:41
How did we allow ourselves to be taken in by this corporate giant. USA government has a long history with Monsanto.
In my province, herbicides like glyphosates have been banned except when required although strict regulations are in place. In other words, it was deemed important to get people used to seeing weeds on lawns and city landscapes rather than spraying nasty herbicides. The bee killing herbicides were banned this summer. I have seen approximately 25 bees busy on the blooms, a sight for sore eyes.
olivier sepval Comment left 30th March 2016 11:11:54
there are other herbicides Bio Control Beloukha kind that can serve the interests of farmers and the environment. We want to produce cleaner with natural fungicides that act on the Defense System of the plants, we must ensure that the Glyphosate does not diminish the immune system of crops. To be continued