Clinical trials of malaria vaccines on infants raise serious concerns over the safety of multiple vaccinations of the very young
Effective implementation of existing measures have eradicated malaria from many countries without using vaccines Dr. Mae-Wan Ho and Prof. Joe Cummins
About 40 percent of the world’s population live in areas with malaria, and an estimated 300-500 million are infected a year; of which 1.5-2.7 m die [1]. In the year 2000, malaria caused nearly 45 million Disability Adjusted Life Years (DALYs), accounting for 13 percent of all DALYs associated with infectious diseases. Malaria is caused by a protozoan (single celled animal) parasite transmitted by blood sucking mosquitoes. There are four species of protozoa that cause malaria; Plasmodium falciparum is responsible for the majority of infections and is the most fatal.
The main strategies for combating malaria include controlling the mosquito vectors with insecticides-treated bed nets, residual insecticides (insecticides that remain active over extended periods) or treating the parasite infection with combination therapy based on artimisinin (a natural product of wormwood discovered in China), and other anti-malarial drugs such as those based on quinine [2] (Two Takes on Malaria, SiS 13/14). These measures have eradicated malaria, especially from Europe and the United States. However, malaria remains a scourge in the tropics and subtropics [1] (Fig. 1), and in recent years, much emphasis has been placed on developing malaria vaccines [3] as an additional measure in fighting malaria.
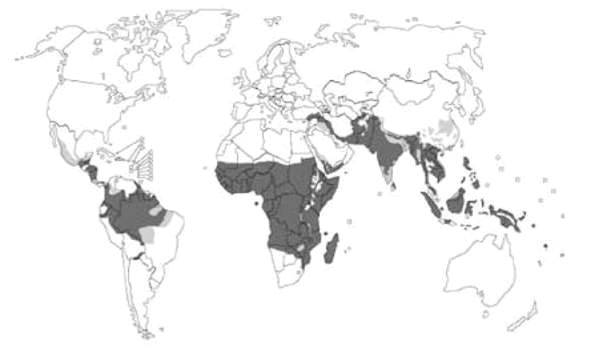
Figure 1. World map of malaria, black, areas where malaria is transmitted, grey, areas with limited risk, white, areas with no malaria (source WHO, 2003)
The Plasmodium parasites that enter the bloodstream are called sporozoites. Sporozoites go to the liver, where they multiply before changing into a different form called merozoites. The merozoites enter into the red blood cells (erythrocytes) to multiply; and this makes the person very sick with symptoms of malaria. A person can look well but still have Plasmodium in the liver in a dormant phase. Weeks or months later, the Plasmodium can leave the liver and enter the bloodstream, and the person will get sick again. P. falciparum causes the most dangerous type of malaria, making people sicker than other Plasmodium species, because there are more of them in the blood. With falciparum malaria, the red blood cells are sticky, so they block the blood vessels [4].
Attenuated sporozoite vaccine
Sporozoites have been attenuated (weakened) with irradiation, and injection of such sporozoites provided complete protection. The production of attenuated sporozoites has been improved and clinical trials will begin in the near future by injecting the vaccine. Genetic attenuation of sporozoites is also used to produce mutant strains unable to complete their life cycle [3]. Attenuated sporozoite vaccines are attractive for protection against malaria but difficult and costly to produce on a global scale.
Subunit vaccines
Synthetic and genetically engineered sub-unit vaccines are usually based on either of two sporozoite surface proteins: circumsporozoite protein (CSP), and thrombospondin related anonymous protein (TRAP) involved in sporozoite motility and invasion of liver cells. The RTS,S vaccines, produced in yeast cells, are made up of the tandem repeat tetrapeptide (R) and the C terminal T-cell epitope (antigenic unit) containing (T) regions of CSP fused to hepatitis B surface antigen (S), plus the unfused S antigen [3]. The vaccine contains an adjuvant.ASO2, oil in water emulsion of the immunostimulants monophosphoryl lipid A and QS-21, a fraction of China bark extract (Quillaia saponara). The RTS,S vaccine is the farthest advanced of the malaria vaccines. There has been extensive clinical testing of this vaccine, including several in infants (see later).
Viral vector vaccines
Pre-red cell stage vaccines based on CSP, TRAP and other liver stage antigens are being developed using viral vector delivery systems [3]. A replication –defective adenovirus strain 35 (Ad35) with chromosome deletions was grown in human embryo cells (PER,C6/55k). A synthetic codon optimized part of the P. falciparum CSP was inserted into the viral vector, and placed under the control of a cytomegalovirus promoter and a simian virus 40 terminator signal. This vaccine is intended to promote immune T cells to establish long lasting protection against malaria infection. The viral vaccine was tested on rhesus macaques monkeys, boosted with the RTS,S/ASO1B vaccine. The test animals’ immunity to malaria persisted at a high level for at least 6 months after vaccination and the vaccine was predicted to have a more durable protection against falciparum malaria in humans [5].
A replication-defective recombinant adenoma virus vector was used to produce a vaccine specific for a surface antigen for the blood stages of falciparum merozoite. The vaccine protected against the liver stages of the parasite as well as the blood stages, and multistage protection and enhanced T cell production was observed in experimental animals [6]. A phaseI/ IIa clinical trial was conducted in Oxford, UK, on healthy local volunteers of two vaccines, FP9/MVA ME-TRAP and PEV3A, active against parasites in the pre-erythrocyte, liver, and blood stages. PEV3A, developed by the Swiss Pevion Biotech company, includes peptides from both the pre-eythrocyte CSP and the blood stage antigen AMA-1, delivered with influenza virosomes, virus-like particles that retain the cell binding and membrane fusion properties of the native virus, but lack the viral genetic material. Antigens from the different stages of the parasite were chemically linked to the surface of the virosome to enhance their immune activity. FP9/MVA ME-TRAP is fowlpox strain FP9 and modified vaccinia virus Ankar (MVA) vector expressing the pre-erthyrocyte antigen TRAP fused to a multi-epitope (ME) string developed by Oxford University, previously taken through phase I/II studies in adults and children in Gambia. Subjects were vaccinated with PEV3A alone or in combination with FP9/MVA ME-TRAP. They found evidence of specific immune responses induced by PEV3A vaccinated volunteers, but no volunteers were completely protected from malaria. PEV3A induced high antibody titres and these antibodies bound parasites in immunofluorescence assays; and sporozoite challenge boosted the vaccine-induced immune response. Immune responses induced by FP9/MVA ME-TRAP were unexpectedly low. A substantial number of the volunteers experienced local pain, as well as general symptoms such as joint pain, muscle pain, headache or malaise [7]. In general, protein vaccines that do not contain viral or other recombinant nucleic acid sequences are preferable, because there is little risk from horizontal gene transfer and recombination that can create more lethal parasites as well as novel viral and bacterial pathogens [8] (see Horizontal Gene Transfer from GMOs Does Happen, SiS 38).Transmission blocking vaccines
Vaccines that block transmission of the malaria parasite to human victims have been developed. Plasmodium vivax is a major cause of malaria in Asia and South America, and protein Pvs 25 from P. Vivax was used as vaccine. The immunized subject produced antisera which was present in the blood meal of a mosquito. The mosquito that ingested such a blood meal prevented it from transmitting the parasite to any further victim. A recombinant vaccine was produced in yeast that went through a phase I clinical trial and no vaccine-related serious adverse events were observed [9].
A recombinant transmission-blocking vaccine directed at Plasmodium falciparum was produced in the bacterium E. coli. The vaccine Pfs 48/45 contains part of the protozoan proteins 48/45, which required four bacterial folding proteins because the vaccine protein had to be folded properly to elicit antibodies. The vaccine protein, folded correctly, was stable and highly active as well as safe in mice, but it has not yet progressed to human subjects [10].The RST,S vaccine is the furthest along to clinical use. It gave satisfactory but short lived protection of adults who had never been exposed to malaria [3]. An efficacy of more than 70 percent was reported in 250 male Gambian adults during the first 2 months of follow-up, but falling to 0 percent in the last 6 weeks [4]. This vaccine has since been trialed on children and infants. Studies with infants and children are considered important because they are the most sensitive group for malaria infection.
A double-blind phase I/IIb randomised trial in Mozambique involved 124 infants [11] assigned to receive three doses either of RTS,S/AS02D or hepatitis B vaccine Engerix B at ages 10 weeks, 14 weeks and 18 weeks, as well as routine immunisations (DTPs/HiB) given at 8, 12, and 16 weeks In the three months after the third dose, there were 68 infections, 22 in RTS,S group, 46 in controls. The adjusted vaccine efficacy was 65.9 percent. However, at the end of the follow-up period, the prevalence of infection was 5 percent in the RTS,S group compared with 8 percent in controls, and not significant. This low prevalence of infection is in sharp contrast to the high incidence of infection over this same period, and was due to the intensity of follow up and treatment promptly applied. In the 6 month period of the safety follow-up, there were17 children with adverse events in each group (15.9 percent); 31 serious events in the RTS,S/AS02D group and 30 serious adverse events in the Energerix-B group. But none was reported as related to vaccination, and no further details were given. Four deaths occurred during the same follow up period, 2 in each group. One in the RTS,S group was due to septic shock, and the remaining due to gastroenteritis and severe dehydration. The interpretation was that: “The RTS,S/AS02D malaria vaccine was safe, well tolerated, and immunogenic in young infants. The findings set the stage for expanded phase III efficacy studies to confirm vaccine efficacy against clinical malaria disease.” Seven authors were declared employees of GlaxoSmithKine Biologicals, the vaccine manufacturer, four owning shares and 2 inventors of patented malarial vaccines Another phase IIb single-center, double-blind controlled trial involved 340 infants in Eagamoya, Tanzania, randomised to receive three doses of either the RTS, S/AS02D vaccine, or the hepatitis B vaccine at 8, 12, and 16 weeks of age. All infants also received a vaccine containing diphtheria and teneus toxoids, whole-cell pertussis vaccine, and conjugated Haemophilius influenzae type b vaccine (DTPs/Hib) [12].During the 6-months period after the third dose of vaccine, the efficacy of the RTS,S/AS02D vaccine against first infection with P. falciparum malaria was 65.2 percent.
At least one serious adverse event occurred in 31 of 170 infants (18 percent) who received the RTS,S/AS02D vaccineand in 42 out of the 170 (24.7 percent) infants who received the hepatitis B vaccine.The most frequent serious adverse event was pneumonia, followed by anaemia, and gastroenteritis. There was one death in the hepatitis B group following severe pneumonia and seizures. The adverse events and death were deemed unrelated to vaccination.
The report concluded that the vaccine had a “promising safety profile” and did not interfere with the immunologic responses to co-administered EPI [WHO’s expanded program of immunization] antigens.
A previous study with AS02A adjuvant showed a 30 percent rate of protection against malaria in children 1 to 4 years, so a more immunogenic adjuvant was used with the vaccine for a study in Kilifi, Kenya, and Korogwe, Tanzania, compared with rabies vaccine in a double-blind, randomized trial [13]. A total of 894 children were recruited; and 809 completed the study.
In the follow up period 32 of 402 receiving the malaria vaccine developed clinical malaria compared with 66 of 407 assigned to the rabies vaccine. So the efficacy was 53 percent. A total of 47 of 447 children receiving RTS,S/ AS01E had one or more serious adverse events (11 percent), compared to 82 of the 227 receiving the rabies vaccine (18 percent). The most frequent adverse events were: pneumonia, gastroenteritis, and respiratory tract infection. There was one death each from malaria vaccine and rabies vaccine. Again, the adverse events and deaths were considered unrelated to vaccination
These recent trials of malaria vaccines on infants in Africa raise serious concerns over safety, not only of the malaria vaccine being tested, but of vaccines in general administered in large numbers to the young and very young. The malaria vaccine is being tested against a background of multiple vaccines, and furthermore, against vaccines with highly controversial safety record such as the hepatitis B vaccine [14, 15].
From 1990 to the end of 2002, the Vaccine Adverse Events Reporting System (VAERS), set up by the Centers for Disease Control and Food and Drug Administration in the United States, received reports of 9 520 serious adverse events in children under one year of age after one dose of hepatitis B vaccine, either alone or with other vaccines; among these were 627 deaths. In the same period, there were 38 600 serious adverse events and 753 deaths over all ages for the hepatitis B vaccine. Clearly deaths among infants less than 1 year after hepatitis B vaccination were much higher than those in adults and older children.
In the malaria vaccine trials, serious adverse events of 10 to 20 percent or more and even deaths in both trial and control groups were routinely dismissed as ‘unrelated to vaccination’, and hence did not even enter into the VAERS statistics.
The level of protection offered by the malaria vaccine was at best 65.9 percent in the follow up period of 3 months after the final dose, and there is no evidence it lasted longer than that. This level of efficacy is insignificant when treatment is promptly applied, and certainly not worth the risks of serious adverse events including death.
Yet, all the trials concluded that the vaccine was safe, and “immunogenic in young infants” and warrant a large scale phase III multi-centre trial.
The trials were all sponsored by the vaccine manufacturer GlaxoSmithKline Biologicals, with its employees making up a large proportion of the co-authors, some of them holding shares in the company and patents for malaria vaccines [11-13]. The potential conflict of interest cannot be ignored. In addition, a large charity, Program for Appropriate Technology in Health (PATH) was listed in two of the three studies [12, 13]. PATH describes itself as a charity [16] funded at US$168 million in 2007, by “foundations, the United States government, other governments, multilateral agencies, corporations, and individuals.” Misguided governments and mega-foundations such as the Bill and Melinda Gates Foundation are complicit in the promotion of these and other even more aggressive vaccines in the pipelines that are of dubious benefit to the countries whose infants are being recruited for clinical trials.
Ivan Tonna at Radcliffe Hospital, Oxford, in the UK, pointed out that vaccines are not cost-effective for developing countries [1]. The worst case scenario of using more and more aggressive vaccines is that it may provoke the evolution of a parasite with a higher virulence. Plasmodium falciparum is well-known for its genetic dexterity. It has multiple stages in its life cycle, each stage expressing a different repertoire of antigens, and many of these exhibit remarkable polymorphisms.
In the case of malaria, existing measures have succeeded in eradicating the disease when they are adequately implemented especially in European countries and the United States. The Roll Back Malaria campaign initiated by WHO [2] has had some notable successes: Vietnam has seen its malaria deaths reduced by 97 percent in five years, and in Kenya, the promotion of bed nets has helped reduce malaria cases significantly. There are safe and affordable alternatives to vaccines [1, 2].Article first published 16/03/09
Comments are now closed for this article