Science of the Organism
Predictions from quantum chemistry calculations and state of the art cryogenic electrospray ionization infrared spectroscopy provide evidence that water exists as pentagonal dodecahedron clusters based on the golden ratio, the magic number woven into the fabric of the universe and consciousness Dr. Mae-Wan Ho
The pentagonal dodecahedron (Figures 1) is a Platonic solid based on the golden ratio (as is the pentagon itself); it has 12 pentagon faces, 20 vertices and 30 edges. Water clusters structured as pentagonal dodecahedra consist of 20 water molecules (H2O)20 connected by hydrogen bonds. This cage structure is one of the building blocks of sI clathrate hyrates, crystalline ice-like solids in which gases (such as methane) and other molecules are trapped [1]. Clathrate hydrates are found naturally in deep oceans and permafrost regions of the earth, raising fears that global warming will release unprecedented amounts of the potent greenhouse gas methane into the atmosphere.
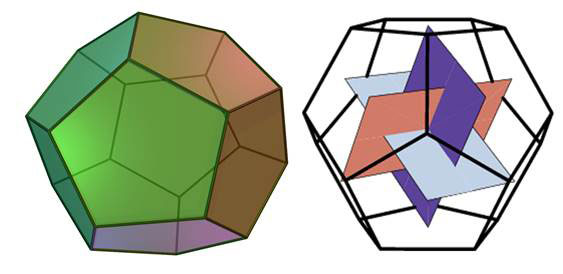
Figure 1 The pentagonal dodecahedron; joining the centres of the faces gives three mutually perpendicular golden rectangles, the length of the sides being in the golden ratio
But even for a well-defined structure like the dodecahedron, the number of possible forms (isomers) is enormous because the H-bonds can join up different neighbouring molecules. Applying the ‘ice rules’ (to include only neutral clusters) to the H atom positions for a fixed O network reduces the number of isomers to 30 026, which is still a lot.
Similarly, a protonated, positively charged dodecahedron cluster H3O+(H2O)20 with an extra hydronium ion, is a centre of attention because 21 was identified as a ‘magic number’ (an unusually strong signal) in the mass spectra of water clusters increasing from 11 to 33; and attributed to its enhanced stability (reviewed in [2]). But the number of possible isomers for H3O+(H2O)20 is even greater than that of (H2O)20.
To find the actual structure that exists, one needs to calculate the potential energy of all possible isomers to pinpoint the ones with the lowest energies and hence most stable. The vibration spectra of these isomers in the infrared region are then predicted and compared with the experimental spectra. Much work has been done since the 1980s but definitive match between theory and experiment is yet to be achieved.
In 2012, Sotiris Xantheas at Pacific Northwest National Laboratory, Richland, Washington, USA, created a database of low-energy pentagonal dodecahedron (H2O)20 and H3O+(H2O)20 from electronic structure calculations, using as starting points previously identified isomers that have the lowest energies, and putting them through several different potential energy computation routines [3]. There were 20 least-energy isomers for (H2O)20, and 55 for H3O+(H2O)20 gleaned from previous work. A hierarchical approach taking advantage of the least-energy isomers already identified provides the best chance of locating the ‘true’ energy minima, and hence the most stable forms that are likely to exist in real life.
Xantheas re-optimized the starting minimum energy structures first by DFT (density function theory) using Becke’s three parameter hybrid function (B3LYP) and Ahlrich’s triple valence polarised TZVP basis set (A basis set is a set of functions, called basis functions, combined linearly as part of a quantum chemical calculation to create molecular orbitals).
The lowest lying 13 (H2O)20 and 7 H3O+(H2O)20 obtained were further used as starting points for optimization by MP2 (Møller–Plesset perturbation theory) with Dunning’s aug-cc-pVDZ (augmented correlation consistent double zeta) basis set. There are numerous quantum chemical computation software packages representing different levels of electronic structure and they are constantly being refined by quantum chemists and other theorists for different molecular systems (see [4-6] for more details).
For (H2O)20, isomer #15 was predicted the global minimum by DFT B3YP with binding energy -231.008 kcal/mol; and also by MP2/aug-cc-pVDZ at a highly energy of -212.096 kcal/mol. Isomer #2 was the global minimum with TIP4P (Transferable Intermolecular Potential with 4 Points, a standard water model used by previous researchers). For comparison the two isomers are presented in Figure 2. A small energy difference - 0.13 kcal/mol - separates the two isomers, but the predicted IR spectra are quite different. Notably, in the harmonic spectra (containing only frequency components in whole number multiples of the fundamental frequency), a band centred around 2 750 cm-1 present in #15 is absent in #2. In order to compare with actual experimental data, however, the spectra need to be corrected for DFT level of theory as well as anharmonicity (deviation from harmonic vibrations), both of which would change the actual positions of many bands in the spectra.
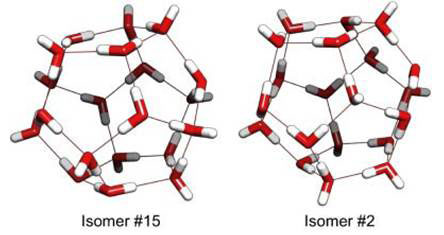
Figure 2 Least energy isomers for dodecahedron clusters with 20 water molecules
For H3O+(H2O)20, the first 7 low-lying isomers with the ASP (Anisotropic Site Potential, used by previous researchers) form a group separated by ~0.6 kcal/mol from ones higher in energy. This same group is reproduced by DFT, separated by ~0.8 kcal/mol from higher energy isomers. The optimal structures obtained with MP2/aug-cc-pVDZ, isomers #5 (global minimum), #3 (first minimum above the global) and #1 (global minimum with the ASP and at the B3LYP/TZVP and B3LYP/augcc- pVDZ) are shown in Figure 3.
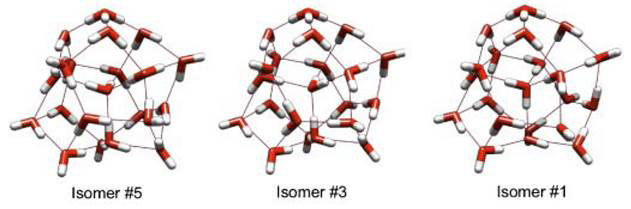
Figure 3 Least energy isomers for protonated dodecahedron clusters with 21 water molecules
All three isomers show 9 free (‘dangling’) OH bonds with the hydronium lying on the surface of the cluster and a fully coordinated water at the centre; i.e., the centre of the cage is filled, and not hollow as in the case of (H2O)20. The harmonic IR spectral obtained with B4LYP/TZVP for the isomers were distinct.
The proton in water could be in the Eigen form H3O+, where the proton is strongly linked by a single bond to the oxygen atom of a water molecule, or in the Zundel form, where it lies midway between the oxygen atoms of two water molecules (H2O - H+ - OH2). Miquel Torrent-Sucarrat and Joseph Anglada at University of Barcelona calculated the IR spectra for the most stable conformers of the H+(H2O)3, H+ (H2O)4 and H+(H2O)21 water clusters, all with the Eigen structure, taking anharmonicity into account [7]. They showed that the O-H stretch vibrations of H3O+ are redshifted (to longer wavelengths) by more than 500 cm-1.
They used the DFT B3LYP exchange-correlation functional with 6-31+G(d) and 6-311++G(3df, 3pd) basis sets to optimize the geometries of the three clusters. The most stable conformer of H+(H2O)21 with the hydronium ion sitting on the surface obtained by previous researchers was used as the initial geometry of the optimization process.
For the H+(H2O)21 cluster, the calculations show that the anharmonic effects produce important redshifts in the computed bands. In the 3 000 to 4 000 cm-1 range, the computed anharmonic IR spectrum is more similar to the experimental spectrum than the harmonic ones. The calculations predict the symmetric and antisymmetric OH stretching of the Eigen signature to appear at ~2 500 and ~2 000 cm-1 , with redshifts larger than 250 and 500 cm-1 respectively. The remaining discrepancies between theoretical and experimental IR spectra were attributed to thermodynamic and dynamic effects and would therefore disappear at very low temperatures.
The problem was that the IR spectra would need to go way down in wave numbers beyond the range accessible in most experimental setups.
The breakthrough came with cryogenic IR spectroscopy with electrospray ionization pioneered at Yale University, New Haven, Connecticut in the United States [8]. Electrospray ionization is a technique used in mass spectrometry where a high voltage is applied to a liquid through a capillary tube, forming a jet from which charged liquid droplets (aerosols) are radially dispersed through electrostatic repulsion. In cryogenic IR spectroscopy, the ions are frozen into stable configurations by cooling to a temperature of 10 K or less. This traps some of the molecules in otherwise short-lived states. An atmosphere enriched in deuterium gas D2 is introduced, causing a D2 molecule to associate with each of the trapped species. The desired D2 adduct can then be separated by its heavier mass and exposed to IR radiation. The temperature increase caused by absorption of IR energy makes the D2 molecule evaporate away. By detecting the lighter fragment arising from D2 loss as the frequency is scanned, the vibrational IR spectrum is obtained with great sensitivity.
Using this new technique, a team led by Mark Johnson at Yale University showed that the magic H3O+(H2O)20 cluster yields a particularly clear spectral signature that with the aid of theoretical predictions, can be traced to specific classes of network sites in the predicted pentagonal dodecahedron H-bonded cage, with the hydronium ion residing on the surface. By cooling the ion down to cryogenic temperatures and tagging it with loosely bound deuterium, they were able to extend the vibrational spectrum over previously unattainable range of 4 000 cm-1 right down to 600 cm-1, and detect the crucial spectral signatures [9].
There is a consensus among theorists that the n=21 cluster has an embedded hydronium ion (H3O)+ on the outside of a puckered dodecahedron cage structure with one interior neutral water molecule (isomer #1, Figure 3). In this arrangement, the hydronium motif donates its three hydrogens to three water molecules in single H-bond acceptor configurations and with their OH groups integrated into the surface network formed by essentially neutral water molecules. Earlier experimental work focussed on the OH stretching bands of the neutral water network that can be accessed with readily available laser sources. Unfortunately, the critical strong bands predicted for the symmetric and
antisymmetric OH stretching of the embedded H3O+ near 2 600 cm−1 were not present in the observed spectra [2], which did not extend beyond 2 000 cm-1.
Since then, theoretical predictions that took account of anharmonicity showed that those bands would be considerably redshifted (see [7] above) to below 2 000 cm-1. And when the range of IR spectrum was extended to below 2 000 cm-1, additional band were indeed observed with the higher resolution that comes with D2 adduction.
The theoretical structures proposed earlier (labelled I) in Figure 4, and the reported spectrum of the cryogenically cooled, D2 tagged H3O+(H2O)20 cluster is compared with that of the H3O+(H2O)3 Eigen cation (Fig 4 A and B respectively). The key bands derived from the OH stretch modes of the surface-embedded hydronium (circled in red) denoted vaH2o+ for antisymmetric OH stretching vibration, were assigned to the broad peak in the experimental spectrum more than 500 cm-1 below the corresponding peaks in the free Eigen cation (from ~2 500-2 600 to ~1 900 cm-1), and the umbrella bending mode of the surface-bound hydronium ion, vumbH2o+ is blue-shifted from ~1 000 to ~1 200 cm-1. In contrast, the intramolecular HOH bending and OH stretching modes of the surface water molecules are not strongly shifted by the introduction of the ion, and appear as a broad envelop spanning 3 000 to 3 700 cm-1. An interesting aspect of the D2 tagged spectrum obtained with cryogenic cooling is that distinct features begin to emerge from the continuous background absorption in the OH stretch region above 3 400 cm-1. For example, water molecules in the dodecahedron that act as double acceptors of hydrogen bonds and a single donor (AAD, circled in orange, A for acceptor and D for donor) form a sharp discrete band at 3 700 cm-1, and a well-defined peak at 3 580 cm-1 is attributed to ADD (circled in turquoise) before the crowded region extending down from 3 450 to 3 000 cm1. In addition, new bands attributed to librations –swaying movements of molecules – are evident from 1 000 down to 600 cm-1.
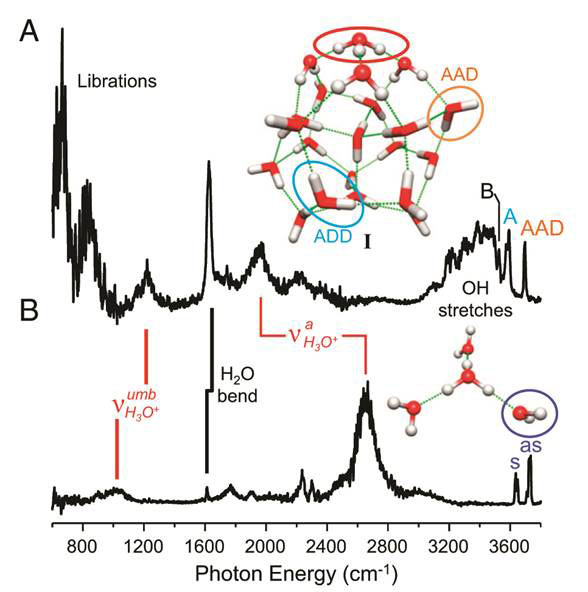
Figure 4 Vibrational spectra A, H3O+(H2O)20 cluster and B, H3O+(H2O)3 Eigen cation; structure I is the supramolecular dodecahedron model of 20 water molecules with the hydronium ion H3O (circled in red) sitting on top (see text for details)
In a new study, the behaviour of the n=20 hydrates of the H3O+ and Cs+ ions are compared [10]. Both hydrates are magic numbers for M+(H2O)n, and thought to be based on a pentagonal dodecahedron motif. An important difference between these ions is that H3O+ is predicted to reside on the surface of the cage whereas Cs+ is sequestered inside.
Figure 5 compares the D2 tagged spectra of H3O+(H2O)20 and Cs+(H2O)20 and the perdeuterated isotopologues D3O +(D2O)20, Cs+(D2O)20. The bands assigned to the antisymmetric hydronium OH stretch (vaH2o+) near 2 000 cm-1 (Fig 5 A highlighted in red) are observed to shift in the heavy isotopologue (Fig. 5B) to a position close to 1 600 cm-1 as expected for the increase in mass. Notably, the bands are absent in the spectra of Cs+(D2O)20 and Cs+(H2O)20 (Fig. 5 c and D respectively).
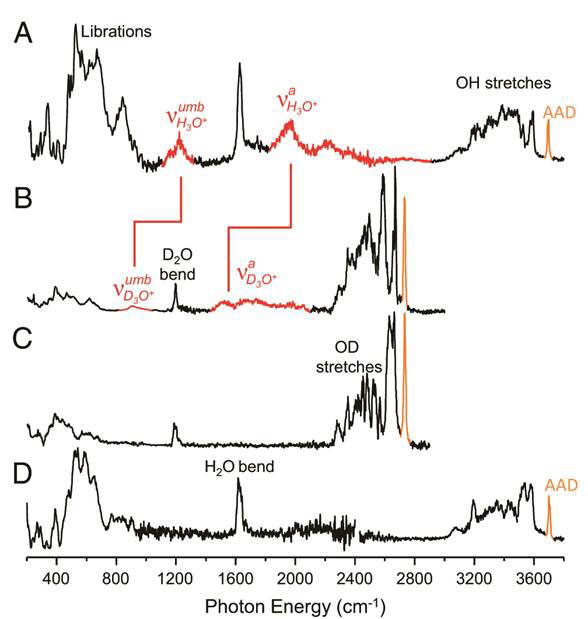
Figure 5 Experimental spectra A, H3O+(H2O)20; B, D3O+(D2O)20; C, Cs+(D2O)20; and D,
Cs+(H2O)20 (see text for further details)
The experimental spectra obtained are so clearly structured that it appears to match the new global minimum identified by Xantheas (isomer #5, Fig. 3) instead of the isomer #1 (Fig. 3).
Figure 6 compares the new least energy structure II with the old structure I, with colour coding to identify the classes of water molecules according to the hydrogen bond configurations and their positions in relation to the hydronium ion.
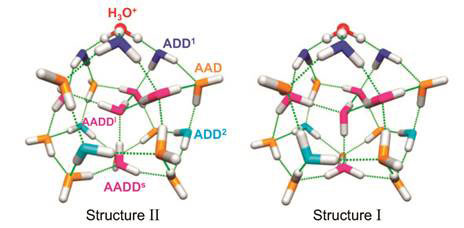
Figure 6 Minimum energy structures with classes of water molecules distinguished by their hydrogen bond configurations and their positions in relation to the hydronium ion
Figure 7 compares the experimental spectra with the predicted harmonic spectra from structure II for both H3O+(H2O)20 and D3O+(D2O)20, together with the bands assigned to specific sites on the dodecahedron structure.
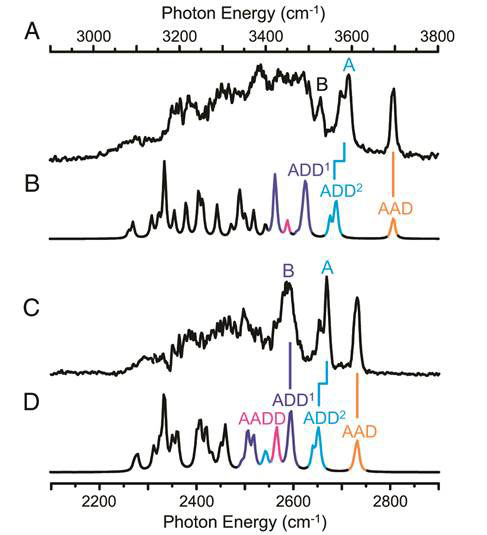
Figure 7 Experimental spectra for H3O+(H2O)20 (A) and D3O+(D2O)20 (C), and their respective harmonic predictions for structure II (B) and (D); the top photon energy scale applies to A and B, the bottom to C and D
The unprecedented structured contained in the new experimental spectra obtained encouraged the team to make further specific assignments of bands to sites for the lower energy part of the spectra. These will no doubt be refined and made unambiguous in future work.
Within the past decade, substantial evidence has emerged that liquid water is structured and quantum coherent, constituting the ‘means, medium, and message of life’ (see [11] Living Rainbow H2O, ISIS publication). The new findings suggest that water is structured according to the golden ratio, a magic number woven into the fabric of the universe and consciousness (see [12] The Story of Phi parts 1-6, SiS 62). They are also consistent with the icosahedral structure containing 280 water molecules proposed by Martin Chaplin in 1999 [13] (see also [14] The Importance of Cell Water, SiS 24), built up from tetrahedrally hydrogen-bonded water molecules surrounding a dodecahedron core.
In a new publication, we propose that spacetime is fractal and quantum coherent in the golden ratio [15]. Could it be that water (and the golden ratio) holds the key to life, the universe and everything?
This paper was reviewed by Martin Chaplin
Article first published 18/03/15
Got something to say about this page? Comment
There are 2 comments on this article so far. Add your comment above.
Eddie. S .Ndlovu Comment left 20th March 2015 00:12:00
This is the news we wanna keeping hearing, makes a lot of sense to me.Gotta tell the world about this news Life is really amazing, water (and the golden ratio) holds the key to life, the universe and everything, that's it Doc no Question mark needed.
Eddie. S .Ndlovu Comment left 20th March 2015 00:12:56
Dr Mae-Wan Ho, this is good news for my brains please read again what you wrote, and comment. This is Jazz.